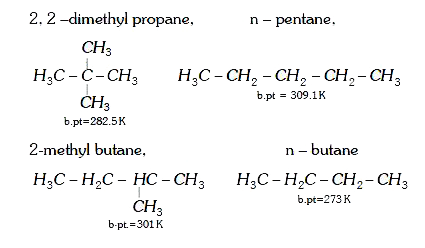A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following in decreasing order of their boiling points. (...
Text Solution
|
- Arrange the following in decreasing order of their boiling points. (...
Text Solution
|
- The correct order of boiling points of 2, 2-dimethylpropane, 2 methylb...
Text Solution
|
- Arrange the following in decreasing order of their boiling points (A) ...
Text Solution
|
- Arrange the following in decreasing order of their boiling points: (I)...
Text Solution
|
- Decreasing order of boiling points of n-Petanol (A), n-Pentane (B), Pe...
Text Solution
|
- The correct order of decreasing boiling points of the following hydro...
Text Solution
|
- Arrange the following in decreasing order of their boiling points. (a)...
Text Solution
|
- (a) n-butane (b) 2-methylbutane (c) -pentane (d) 2 , 2 The order in wh...
Text Solution
|