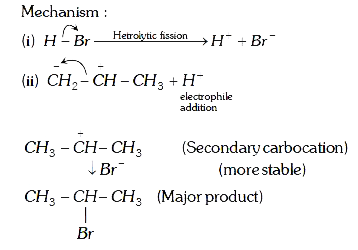A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What type of reaction is involved is the reaction CH(2) - CH - CH(3) +...
Text Solution
|
- What is the major bromination product in the following reaction ? CH...
Text Solution
|
- CH(3)-underset(CH(3)) underset(|) (C)= CH(2) underset("Peroxide") over...
Text Solution
|
- The reaction CH(2)=CH-CH(3)+HBr rarr CH(3)-overset(Br)overset(|)(C )HC...
Text Solution
|
- 3-मेथीलब्यूटेन-2-ऑल की HBr से क्रिया निम्न प्रकार से है- {:(" ...
Text Solution
|
- Identify the type of the following reaction of carbon compounds CH(3)-...
Text Solution
|
- CH(3)-CH= CH-CH(3)+ Br(2) to CH(3)-underset(Br)underset(|)CH-underset(...
Text Solution
|
- The reaction of CH(3)CH=CH- with HBr gives :
Text Solution
|
- Complete the following Reaction CH(3)-CH=CH(2)+HBr C Cl(3)-CH=CH(2...
Text Solution
|