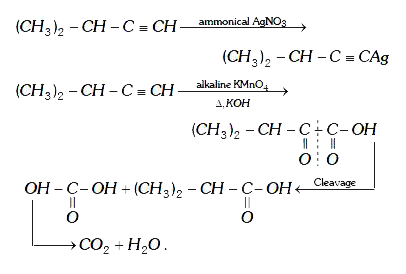A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A compound X (C5H8) reacts with ammoniacal AgNO3 to give a white preci...
Text Solution
|
- Which of the following does not give white precipitate with ammoniacal...
Text Solution
|
- A compound C5H8 which gives white ppt. with ammonical AgNO3 .A give (C...
Text Solution
|
- A gas decolourises alkaline KMnO(4) solution but does not give precipi...
Text Solution
|
- A compound X (C5H8) reacts with ammoniacal AgNO3 to give a white preci...
Text Solution
|
- Butyne-1 on oxidation with hot alkaline KMnO4 would give
Text Solution
|
- A compound (C(5)H(8)) reacts with ammoniacal AgNO(3) to give a white p...
Text Solution
|
- निम्न में से कौनसी अमोनियामय AgNO(3) के साथ सफेद अवक्षेप नहीं देगा-
Text Solution
|
- एक यौगिक C5 H8 जो अमोनियामय AgNO3 के साथ सफेद अवक्षेप देता है A गर्म ए...
Text Solution
|