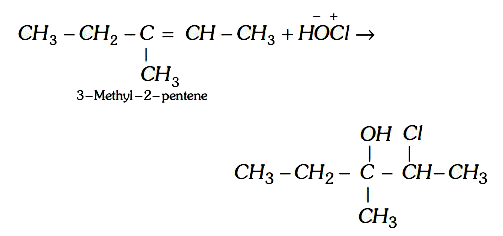A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The predominant product formed when 3- methyl -2-pentene reacts with H...
Text Solution
|
- Ethene reacts with HOCl to form
Text Solution
|
- The predominant product formed when 3- methyl -2- pentene reacts with ...
Text Solution
|
- What would be the product when 2-pentene reacts with HBr-
Text Solution
|
- 3-Methyl-2-pentene on reaction with HOCl gives-
Text Solution
|
- 2-methyl-2-pentenal
Text Solution
|
- 4-methyl-3-pentenal
Text Solution
|
- 3-methyl-1-pentene
Text Solution
|
- The predominant product formed when 3- methyl -2-pentene reacts with H...
Text Solution
|