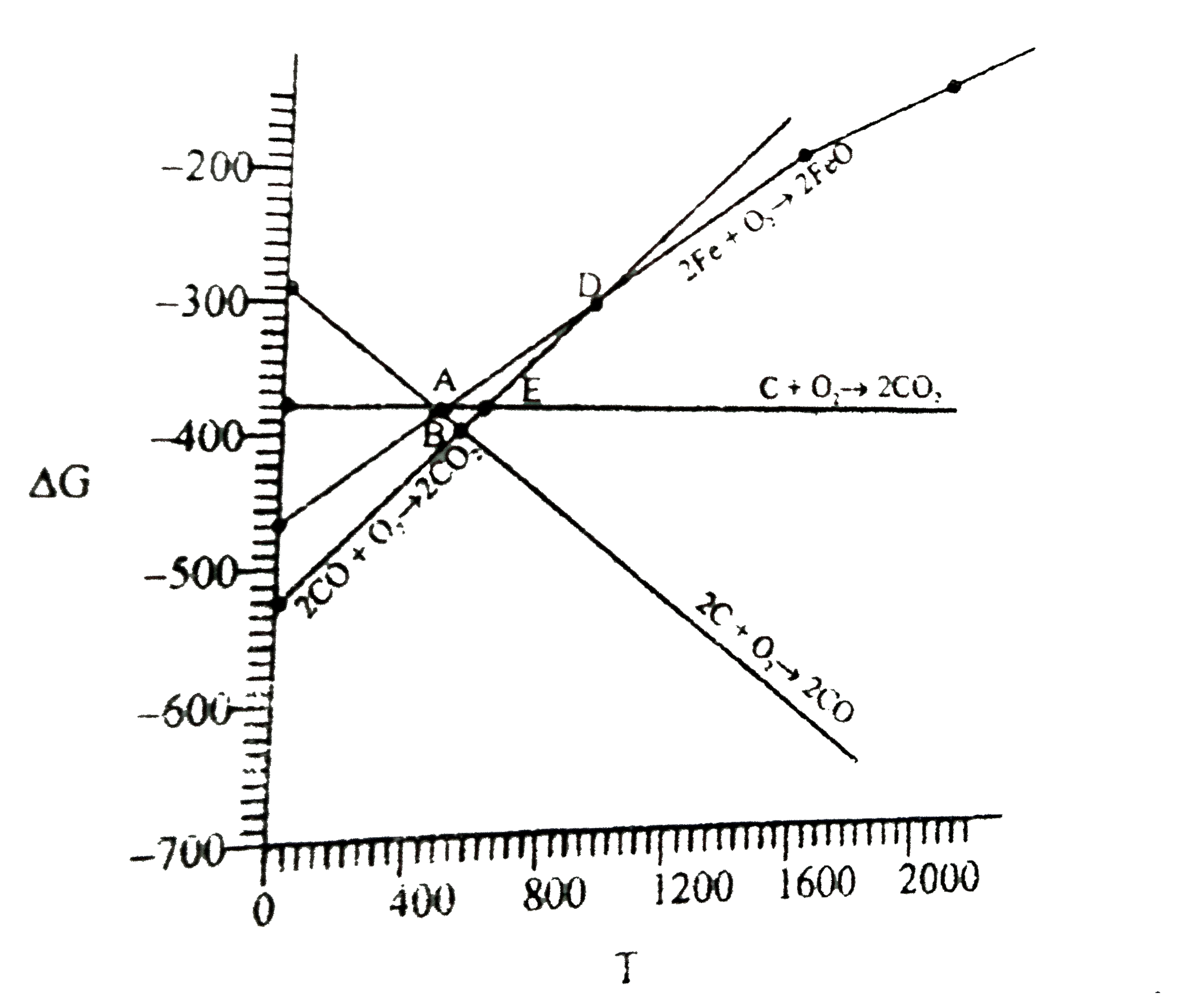
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Choose the correct option of temperature at which carbon reduced FeO t...
Text Solution
|
- Indicate the temperature at which carbon can be used as a reducing age...
Text Solution
|
- Choose the correct option of temperature at which carbon reduced FeO t...
Text Solution
|
- Choose the correct option of temperature at which carobon reduces FeO ...
Text Solution
|
- Choose the correct option of temperature at which carbon reduces FeO t...
Text Solution
|
- Indicate the temperature at which carbon can be used as a reducing age...
Text Solution
|
- किस ताप पर कार्बन FeO को अपचयित करता है ?
Text Solution
|
- কোন্ উম্নতায় কার্বন, FeO-কে বিজারিত করে Fe ও Co উৎপন্ন করে
Text Solution
|
- निर्देशः चित्र के आधार पर 11-13 प्रश्नों के उत्तर दीजिए। उस ताप ...
Text Solution
|