A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
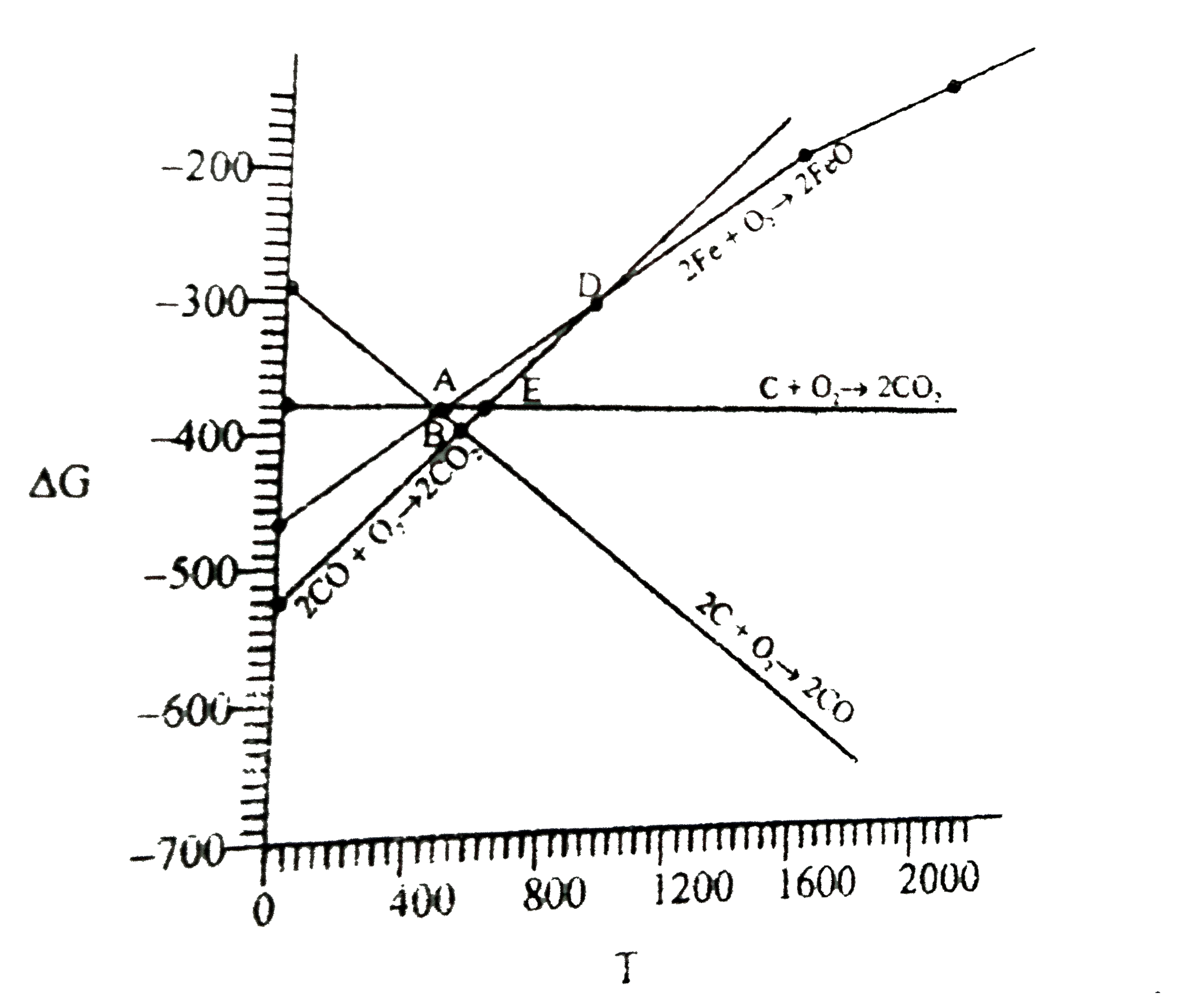
- For the reduction of FeO at the temperature corresponding to point D, ...
Text Solution
|
- For the reduction of FeO at the temperature corresponding to point D, ...
Text Solution
|
- For the reduction of FeO at the temperature corresponding to point D, ...
Text Solution
|
- Correct statement about FeO at room temperature
Text Solution
|
- Select the correct statement in the case of reduction of FeO at a temp...
Text Solution
|
- Which of the following statement is correct regarding Reduction Potent...
Text Solution
|
- Correct statement about FeO at room temperature
Text Solution
|
- D বিন্দু দ্বারা প্রকাশিত উষ্ণতায় FeO-এর বিজারণের ক্ষেত্রে সঠিক বিবৃতি...
Text Solution
|
- कुछ ऑक्साइडों के निर्माण के लिए एलिंघम चित्र का अध्ययन कीजिए तथा निम्न...
Text Solution
|