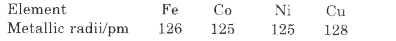A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Metallic radii of some transition elements are given below. Wh...
Text Solution
|
- Metallic radii of some transitions element are given below. Which of t...
Text Solution
|
- Metallic radii of some transitions element are given below. Which of t...
Text Solution
|
- Metallic radii of some transition elements are given below. Which of t...
Text Solution
|
- Name the transition elements with the highest and the lowest density.
Text Solution
|
- Metallic radii of some transition elements are given below. Which of t...
Text Solution
|
- Name the transition element that has the highest density.
Text Solution
|
- Below are the metallic radii of some of the articular elements. Which ...
Text Solution
|
- Metallic radii of some transition elements are given below. Wh...
Text Solution
|