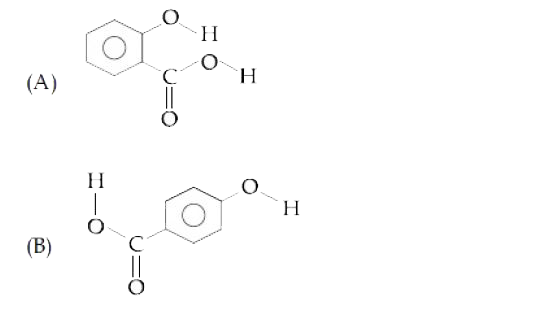
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following molecules and statements related to them : ...
Text Solution
|
- Statement-I: Chloropropane has higher boiling point than chloroethane....
Text Solution
|
- In the given circuit the point A is 9 V higher than point B. Which of ...
Text Solution
|
- , (A) B is less water soluble than A (B) B is more crystalline in ...
Text Solution
|
- Consider the following molecules and statements related to them : (B) ...
Text Solution
|
- Water has a higher boiling point than hydrogen sulfide. Why?
Text Solution
|
- निम्नलिखित के बारे में बताइए : (क) ईधर के क्वथनांक अल्कोहल से काफी क...
Text Solution
|
- (A) : Water has more boiling point than that of hydrgen fluoride. (R):...
Text Solution
|
- , (A) B is less water soluble than A (B) B is more crystalline in natu...
Text Solution
|