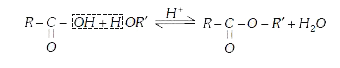A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In esterification, OH^(-) ion for making H(2)O comes from
Text Solution
|
- Basic nature of H(3)O^(+),H(2)O and OH^(-) is in order
Text Solution
|
- H(3)BO(3) + 2H(2)O hArr H(3)O^(+) + [B(OH)(4)]^(-). The additon of w...
Text Solution
|
- HA+OH^(-) to H(2)O+A^(-)+q(1) kJ H^(+)+OH^(-) to H(2)O+ q(2) kJ The en...
Text Solution
|
- In the reaction [A (H(2)O)(6)]^(3+)+H(2)O hArr [Al(H(2)O)(5)OH]^(2+)...
Text Solution
|
- In esterification, OH^(-) ion for making H(2)O comes from
Text Solution
|
- Enthalpy of dissociation of H(2)O molecules into H^(+) and OH^(-) ions...
Text Solution
|
- निम्नलिखित अभिक्रिया को आयन-इलेक्ट्रान विधि द्वारा सन्तुलित कीजिए- C...
Text Solution
|
- निम्नलिखित समीकरण को आयन-इलेक्ट्रॉन विधि के द्वारा सन्तुलित करो- Br(...
Text Solution
|