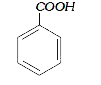
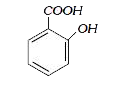
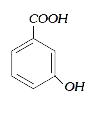
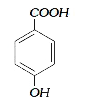
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following aromatic acids is most acidic?
Text Solution
|
- Which of the following aromatic acids has lowest K(a) ?
Text Solution
|
- Which aromatic acid among the following is weaker than simple benzoic ...
Text Solution
|
- Which of the following aromatic acid is most acidic (lowest pK(a) valu...
Text Solution
|
- Which of the following aromatic acids is most acidic?
Text Solution
|
- Which of the following aromatic acids is most acidic?
Text Solution
|
- Which of the following aromatic acids is most acidic?
Text Solution
|
- निम्न ऐरोमैटिक यौगिकों में से सबसे प्रबल अम्ल है- ...
Text Solution
|
- Which of the following aromatic acids is most acidic?
Text Solution
|