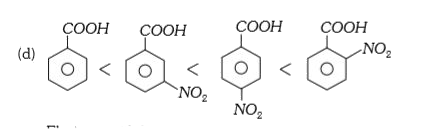
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(...
Text Solution
|
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(2) C...
Text Solution
|
- Arrange in order of decreasing reactivity in electrophilic addition: ...
Text Solution
|
- Consider the acidity of the carboxylic acids : (a) PhCOOH (b) o-NO(2)C...
Text Solution
|
- Consider the acidity of the carboxylic acids I. PhCOOH II. O - NO(...
Text Solution
|
- Arrange the following in order of increasing acidity: (i) Propanoic ac...
Text Solution
|
- Consider the acidity of the carboxylic acids : (i) PHCOOH" "(ii) o-NO(...
Text Solution
|
- The correct order of increasing strength of compounds (1) CH(3) COOH...
Text Solution
|
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(...
Text Solution
|