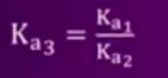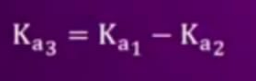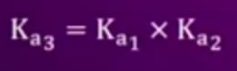A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2022-Question
- Density order in Be,Mg,Ca,Sr,Ba,Ra
Text Solution
|
- 99.9% of pure Hydrogen is prepared by?
Text Solution
|
- Find the relationship between (Ka)1, (Ka)2,(Ka)3 for
Text Solution
|
- Match the column I with column II
Text Solution
|
- A = [Ni(en)3]^(2+), B =[ Ni(NH3)6]^(2+), C = [Ni(H2O)6]^(2+), CFSE ene...
Text Solution
|
- Find the product P.
Text Solution
|
- Same masses of two solutes X and Y are added to two samples of same am...
Text Solution
|
- From 5th transition state to 1st transition in a H-atom sample. Maximu...
Text Solution
|
- How many isomers (including sterio isomers) are there for mono bromina...
Text Solution
|
Text Solution
|
- High purity H2 can be produced by:
Text Solution
|
- Find the molar ratio of gas present in two different container having ...
Text Solution
|
- An electron is present in the 4^(th) excited in H-atom. When it jumps ...
Text Solution
|
- The product formed in the following reaction BeCl2 + LiAlH4
Text Solution
|
- Which of the following used to prevent decomposition of H2O2
Text Solution
|
- Formation of inorganic benzene requires 6 equivalent of X and 3 equiva...
Text Solution
|
- If benzene reacts with Cl2 in the sunlight what is formed and no.of H ...
Text Solution
|
- Liquation process is applicable for metals having?
Text Solution
|
- Which of the following is not aromatic?
Text Solution
|
- If stearic acid and polyethylene glycol reacts then which of the follo...
Text Solution
|