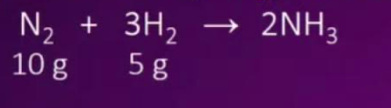A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2022-Question
- Which pair among the following is colourless.
Text Solution
|
- Consider the complex [Fe(OH)6]^(3-) Which act as an inner orbital comp...
Text Solution
|
- Find the limiting reagent and moles of NH3 produced .
Text Solution
|
- The magnitude of change in oxidation state of manganese in KMnO4 in fa...
Text Solution
|
- Which of the following pairs will give different products on ozonolysi...
Text Solution
|
- Find C.
Text Solution
|
- Find A and B respectively.
Text Solution
|
- Which of the following is hypnotic drug?
Text Solution
|
- K(sp) of PbS is given as 9 xx 10^(-30) at given temperature. Its solub...
Text Solution
|
- Which of the following pairs will have one of the compounds having odd...
Text Solution
|
- Find the number of lone pairs present in following species: SCl2, ClF3...
Text Solution
|
- Identify the products formed in the following reaction:
Text Solution
|
- Which of the following is the strongest Bronsted base?
Text Solution
|
- Which of the following are herbicides?
Text Solution
|
- In 5% w/V NaCl solution, we add albumin of egg and stir well. The resu...
Text Solution
|
- The first ionization energy of Na,Mg,Si are 496 , 737 and 786 kj/mol. ...
Text Solution
|
- Calculate the ratio of energy in an H-atom when electron jump from inf...
Text Solution
|
- The product B is :
Text Solution
|
- What is gangue?
Text Solution
|
- Product for the given reaction is: Zn + NaOH
Text Solution
|