Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2022-CHEMISTRY (SECTION-3)
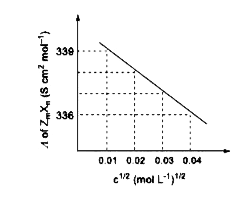
- Consider the strong electrolytes Z(m)X(n) ,U(m)Y(p) and V(m)X(n).Limit...
Text Solution
|
- Match the rate expressions in LIST-I for the decomposition of X with t...
Text Solution
|
- LIST-I contains compounds and LIST-II contains reactions Match e...
Text Solution
|
- LIST-I contains metal species and LIST-II contains their properties. ...
Text Solution
|
- Match the compounds in LIST-I with the observations in LIST-II, and ch...
Text Solution
|