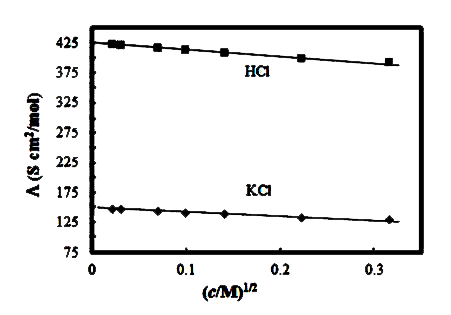
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE PAPER 2023 TERM I-SECTION E
- The molar conductivity of CH(3)COOH at infinite dilution is 390 Scm^...
Text Solution
|
- Why does the cell potential of mercury cell remain constant throughout...
Text Solution
|
- Write the reaction occurring at anode and cathode and the products of ...
Text Solution
|
- The standard free energy change of the reaction Cu^(2+)+Sn(s)rarrCu(s)...
Text Solution
|
- All Cu(II) halides are known except the iodide. The reason for it is t...
Text Solution
|
- Why permanganate titrations in presence of hydrochloric acid are unsa...
Text Solution
|
- D and F block introduction|Properties of d & f block elements metallic...
Text Solution
|
- What is the effect of increasing pH on K2Cr2O7 solution?
Text Solution
|