Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE PAPER 2023 TERM I-SECTION D
- Strengthening the Foundation: Chargaff Formulates His "Rules" any p...
Text Solution
|
- Strengthening the Foundation: Chargaff Formulates His "Rules" any p...
Text Solution
|
- Strengthening the Foundation: Chargaff Formulates His "Rules" any p...
Text Solution
|
- Strengthening the Foundation: Chargaff Formulates His "Rules" any p...
Text Solution
|
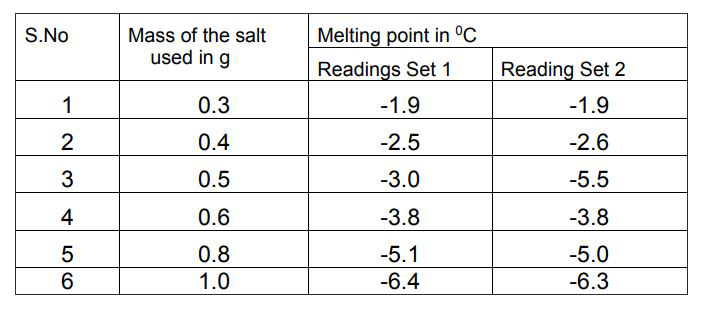
- Henna is investigating the melting point of different salt solutions. ...
Text Solution
|
- Henna is investigating the melting point of different salt solutions. ...
Text Solution
|