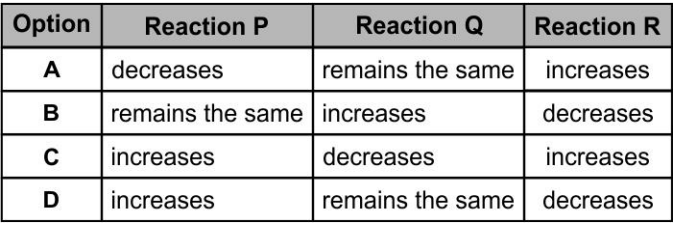
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-Additional Practice Questions-Question
- The yellow colour of turmeric changes to red on addition of soap solut...
Text Solution
|
- During the electrolytic refining of copper what happens at the anode?
Text Solution
|
- Identify the endothermic reaction(s) among the following: (P) 6 CO2 ...
Text Solution
|
- Ashok has written the following reactions to show how metals can be ob...
Text Solution
|
- The following reactions are carried out in open vessels. (P) 2Cu(s)+ ...
Text Solution
|
- A solution of a base with pH 12.1 is given. Which of the following c...
Text Solution
|
- One mole of which of the following compounds requires 2 moles of hydro...
Text Solution
|
- Q. no 17 is Assertion - Reasoning based questions. These consist of ...
Text Solution
|
- Diana prepared a cake by two methods. Method i) She added baking sod...
Text Solution
|
- Compare the stability of a neutral sodium atom and a positive sodium i...
Text Solution
|
- Observe the two chemical equations given below. (P) Ca(OH)2 +HNO3 ri...
Text Solution
|
- The Thermit process is used for repairing cracks in railway tracks on ...
Text Solution
|
- Prasad has a saturated alcohol X of chemical formula C4H9OH. (a) Wri...
Text Solution
|
- An unsaturated hydrocarbon P has the chemical formula C4H6. (a) Writ...
Text Solution
|
- Pure gold is very soft and therefore not suitable for making jewellery...
Text Solution
|
- Pure gold is very soft and therefore not suitable for making jewellery...
Text Solution
|