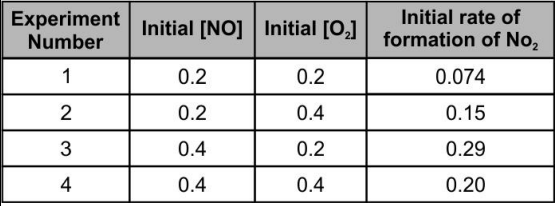
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-Additional Practice Questions-Question
- Which of the following reaction mechanism is not involved in the given...
Text Solution
|
- The graph below shows the observed standard electrode potential of som...
Text Solution
|
- Kamlesh was conducting an experiment to figure out the rate equation o...
Text Solution
|
- The image below shows electrolysis of an electrolyte using a DC voltag...
Text Solution
|
- For a certain reaction X, rate = 0.7Z(AB)e^(-(EA)/(RT)). It is seen ...
Text Solution
|
- A metal ion M^(n+) forms a complex ion of formula [ML2]^((n−4)+) where...
Text Solution
|
- The image below shows different benzene derivates that give mononitrat...
Text Solution
|
- The rate constants of a reaction at 400 K and 600 K are 5 times 10^(-3...
Text Solution
|
- The compound [Co(NH3)5Cl]SO4 is isomeric with the compound [Co(NH3)5SO...
Text Solution
|
- The image below shows an experimental setup to prepare an organic prod...
Text Solution
|
- During protein synthesis in cells, amino acids condense (in the presen...
Text Solution
|
- Zirconium (Zr, Atomic number 40) and Hafnium (Hf, Atomic number 72) ar...
Text Solution
|
- Which of the following would be among the products of the reactions be...
Text Solution
|
- Given below are two statements labelled as Assertion (A) and Reason (R...
Text Solution
|
- Given below are two statements labelled as Assertion (A) and Reason (R...
Text Solution
|
- Given below are two statements labelled as Assertion (A) and Reason (R...
Text Solution
|
- Given below are two statements labelled as Assertion (A) and Reason (R...
Text Solution
|
- At high temperatures, ethyl chloride produces HCl and ethylene by the ...
Text Solution
|
- Pineapple contains a protease enzyme that breaks down proteins. If you...
Text Solution
|
- The chain structure of Lysine is shown below. (i) Based on the st...
Text Solution
|