Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-Additional Practice Questions-Question
- Pineapple contains a protease enzyme that breaks down proteins. If you...
Text Solution
|
- The chain structure of Lysine is shown below. (i) Based on the st...
Text Solution
|
- 2-bromooctane reacts with alcoholic NaOH to give 2-octanol as shown be...
Text Solution
|
- (a) Which of the following two compounds has a chiral centre? (b)...
Text Solution
|
- (i) The complex [PtCl2(NH3)2] has two isomers whereas [CoCl4]^(2-) doe...
Text Solution
|
- The half equation for a redox reaction represents an equilibrium betwe...
Text Solution
|
- A first order reaction is found to have a half-life of 1.15 times 10^4...
Text Solution
|
- Esterification of a carboxylic acid with an alcohol in the presence of...
Text Solution
|
- (a) Is benzaldehyde less or more reactive to electrophilic substitutio...
Text Solution
|
- In 20th century, German scientist Werner succeeded in clarifying the s...
Text Solution
|
- Suman took two glasses of water from a water filter. She cools one gla...
Text Solution
|
- The image below shows the effect of acid and base on the aqueous ethyl...
Text Solution
|
- A mixture of 0.5 moles acetaldehyde and 0.5 moles diethyl ketone is tr...
Text Solution
|
- (a) Show steps to convert nitrobenzene to phenol. (b) The table belo...
Text Solution
|
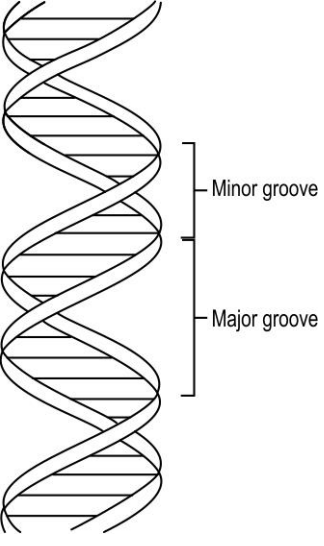
- The image below shows the double helix structure of a DNA. (i) Th...
Text Solution
|
- During a titration, 240 ml of NaOH reacted completely with 100 ml of H...
Text Solution
|
- One of the most common cells that's been used in our daily life is Dur...
Text Solution
|
- Imagine you are in a chemistry lab and the teacher is explaining the e...
Text Solution
|
- The image below shows the boiling point of first seven straight chain ...
Text Solution
|
- (i) Write the outer shell electronic configuration of an element with ...
Text Solution
|