Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2023-Question
- Given below are two statements: Statement I: Lithium and magnesium d...
Text Solution
|
- The titration curve of weak acid vs strong base with phenolphthalein a...
Text Solution
|
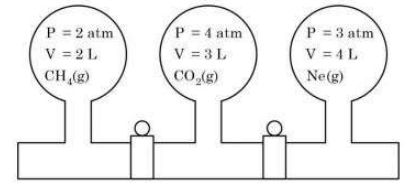
- Three bulbs are filled with CH(4), CO(2) and Ne as shown in the pictur...
Text Solution
|
- The vapour pressure vs. temperature curve for a solution solvent syste...
Text Solution
|
- When a 60 W electric heater is immersed in a gas for 100s in a content...
Text Solution
|
- Molar mass of the hydrocarbon (X) which on ozonolysis consumes one mol...
Text Solution
|
- The number of following factors which affect the percent covalent char...
Text Solution
|
- 0.5 g of an organic compound (X) with 60% carbon will produce times 10...
Text Solution
|
- The number of given statement/s which is/are correct is The stronge...
Text Solution
|
- The number of following statement /s which is/are incorrect is (A) ...
Text Solution
|
- XeF(4) reacts with SbF(5) to form [XeFm]^(n+)[SbFy]^(z-) m+ n+ y+z...
Text Solution
|
- Which of these reactions is not a part of breakdown of ozone in strato...
Text Solution
|
- In Hall - Heroult process, the following is used for reducing Al2O3:-
Text Solution
|
- Which of the following can reduce decomposition of H2O2, on exposure t...
Text Solution
|
- Given below are two statements: Statement I: In redox titration, the i...
Text Solution
|
- The product (P) formed from the following multistep reaction is :
Text Solution
|
- The statement's which are true about antagonists from the following is...
Text Solution
|
- Major product 'P' formed in the following reaction is :
Text Solution
|
- Match List I with List : Choose the correct answer from the option...
Text Solution
|
- Arrange the following gases in increasing order of van der Waals const...
Text Solution
|