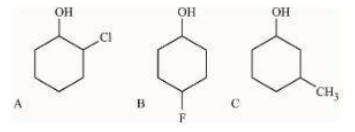
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2023-Question
- Which of the following complexes will exhibit maximum attraction to an...
Text Solution
|
- Match List I with List II 1-Bromopropane is reacted with reagents in...
Text Solution
|
- Given below are two statements, one is labelled as Assertion A and the...
Text Solution
|
- In the wet tests for detection of various cations by precipitation , B...
Text Solution
|
- What happens when methane undergoes combustion in systems A and B resp...
Text Solution
|
- Compound A from the following reaction sequence is :
Text Solution
|
- Given below are two statements related to Ellingham diagram: Stateme...
Text Solution
|
- Given below are two statements : Statement I : Tropolone is an aroma...
Text Solution
|
- The naturally occurring amino acid that contains only one basic functi...
Text Solution
|
- Identify the correct order of standard enthalpy of formation of halide...
Text Solution
|
- Match List I with List II Choose the correct answer from the opti...
Text Solution
|
- The major product for the folllowing reaction is :
Text Solution
|
- The correct group of halide ions which can be oxidised by oxygen in ac...
Text Solution
|
- Which of the following are the Green house gases ? A. Water vapour ...
Text Solution
|
- Better method for preparation of BeF2, among the following is
Text Solution
|
- Given below are two statements, one is labelled as Assertion A and the...
Text Solution
|
- Given below are two statement , one is labelled as Assertion A and the...
Text Solution
|
- The total number of stereoisomers for the complex [Cr ("ox")2ClBr]^(3-...
Text Solution
|
- Given below are two statements: Statement I : SO2 and H2O both posse...
Text Solution
|
- See the following chemical reaction ; Cr(2)O(7)^(2-)+ XH^+ 6Fe^(2+...
Text Solution
|