Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2023-Question
- In Carius tube, an organic compound ‘X’ is treated with sodium peroxid...
Text Solution
|
- The difference in the oxidation state of Xe between the oxidised produ...
Text Solution
|
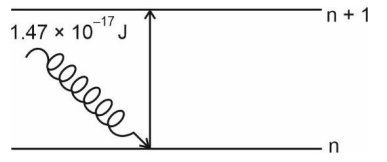
- The electron in the n^th orbit of Li^(2+) is excited to (n + 1) orbit ...
Text Solution
|
- For a metal ion, the calculated magnetic moment is 4.90 BM. This metal...
Text Solution
|
- In alkaline medium, the reduction of permanganate anion involves a gai...
Text Solution
|
- A(g) harr 2B(g)+ C(g) For the given reaction, if the initial pressure...
Text Solution
|
- The number of endothermic process/es from the following is . A. I2(g)...
Text Solution
|
- The specific conductance of 0.0025 M acetic acid is 5 xx 10^(–5) S cm^...
Text Solution
|
- The number of incorrect statement/s from the following is . A. The suc...
Text Solution
|
- The number of molecules from the following which contain only two lone...
Text Solution
|
- An aqueous solution of volume 300 cm^3 contains 0.63 g of protein. The...
Text Solution
|
- Given below are two statements. one is labelled as Assertion A and the...
Text Solution
|
- One mole of P4, reacts with 8 moles of SOCI2 to give 4 moles of A, x m...
Text Solution
|
- What weight of glucose must be dissolved in 100 g of water to lower th...
Text Solution
|
- Given below are two statements : Statement I : Ethene at 333 to 343K a...
Text Solution
|
- Which one of the following pairs is an example of polar molecular soli...
Text Solution
|
- Given below are two statements, one is labelled as Assertion A and the...
Text Solution
|
- Compound 'B' is
Text Solution
|
- Product [X] formed in the above reaction is:
Text Solution
|
- Match List I with List II Choose the correct answer from the option...
Text Solution
|