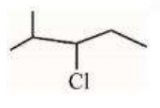
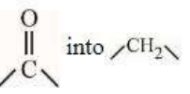A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2023-Question
- The number of molecules from the following which contain only two lone...
Text Solution
|
- An aqueous solution of volume 300 cm^3 contains 0.63 g of protein. The...
Text Solution
|
- Given below are two statements. one is labelled as Assertion A and the...
Text Solution
|
- One mole of P4, reacts with 8 moles of SOCI2 to give 4 moles of A, x m...
Text Solution
|
- What weight of glucose must be dissolved in 100 g of water to lower th...
Text Solution
|
- Given below are two statements : Statement I : Ethene at 333 to 343K a...
Text Solution
|
- Which one of the following pairs is an example of polar molecular soli...
Text Solution
|
- Given below are two statements, one is labelled as Assertion A and the...
Text Solution
|
- Compound 'B' is
Text Solution
|
- Product [X] formed in the above reaction is:
Text Solution
|
- Match List I with List II Choose the correct answer from the option...
Text Solution
|
- The magnetic moment is measured in Bohr Magneton (BM). Spin only magne...
Text Solution
|
- Given below are two statements, one is labelled as Assertion A and the...
Text Solution
|
- A solution is prepared by adding 2 g of "X" to 1 mole of water. Mass p...
Text Solution
|
- For a chemical reaction A+B to "Product", the order is 1 with respect ...
Text Solution
|
- Alkali metal from the following with least melting point is:
Text Solution
|
- Compound from the following that will not produce precipitate on react...
Text Solution
|
- Which hydride among the following is less stable?
Text Solution
|
- If Ni^(2+) is replaced by Pt^(2+) in the complex [NiCl2Br2]^(2-), whic...
Text Solution
|
- Which of the following compounds is an example of Freon?
Text Solution
|
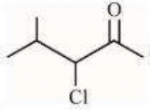 can be subjected to Wolft-Kishner reduction to give
can be subjected to Wolft-Kishner reduction to give