Similar Questions
Explore conceptually related problems
Recommended Questions
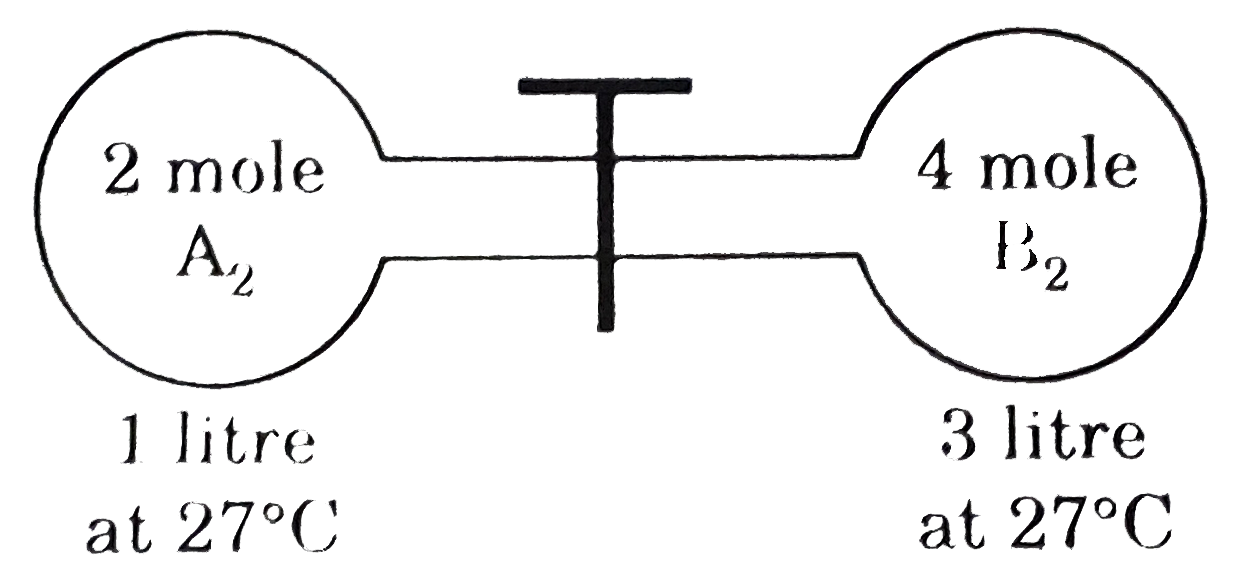
- The gas A2 in the left flask is allowed to react with gas B2 present i...
Text Solution
|
- The gas A(2) in the left flask allowed to react with gas B(2) present ...
Text Solution
|
- A(2)(g)+B(2)(g)hArr 2AB(g), equilibrium constant of the given reaction...
Text Solution
|
- Given the reaction between 2 gases represented by A2 and B2 to give th...
Text Solution
|
- At given temperature the reaction A2(g)+B2(g)hArr2AB(g) was started wi...
Text Solution
|
- A2(g)+B2(g)<implies2AB(g) , equilibrium constant of the given reaction...
Text Solution
|
- When A(2) and B(2) are allowed to react, the equilibrium constant of t...
Text Solution
|
- The equilibrium constant Kc of the reaction, A2(g) +B2(g) hArr 2AB(g) ...
Text Solution
|
- A2 (g) + B2(g) hArr 2AB(g) . The equilibrium constant of above reactio...
Text Solution
|