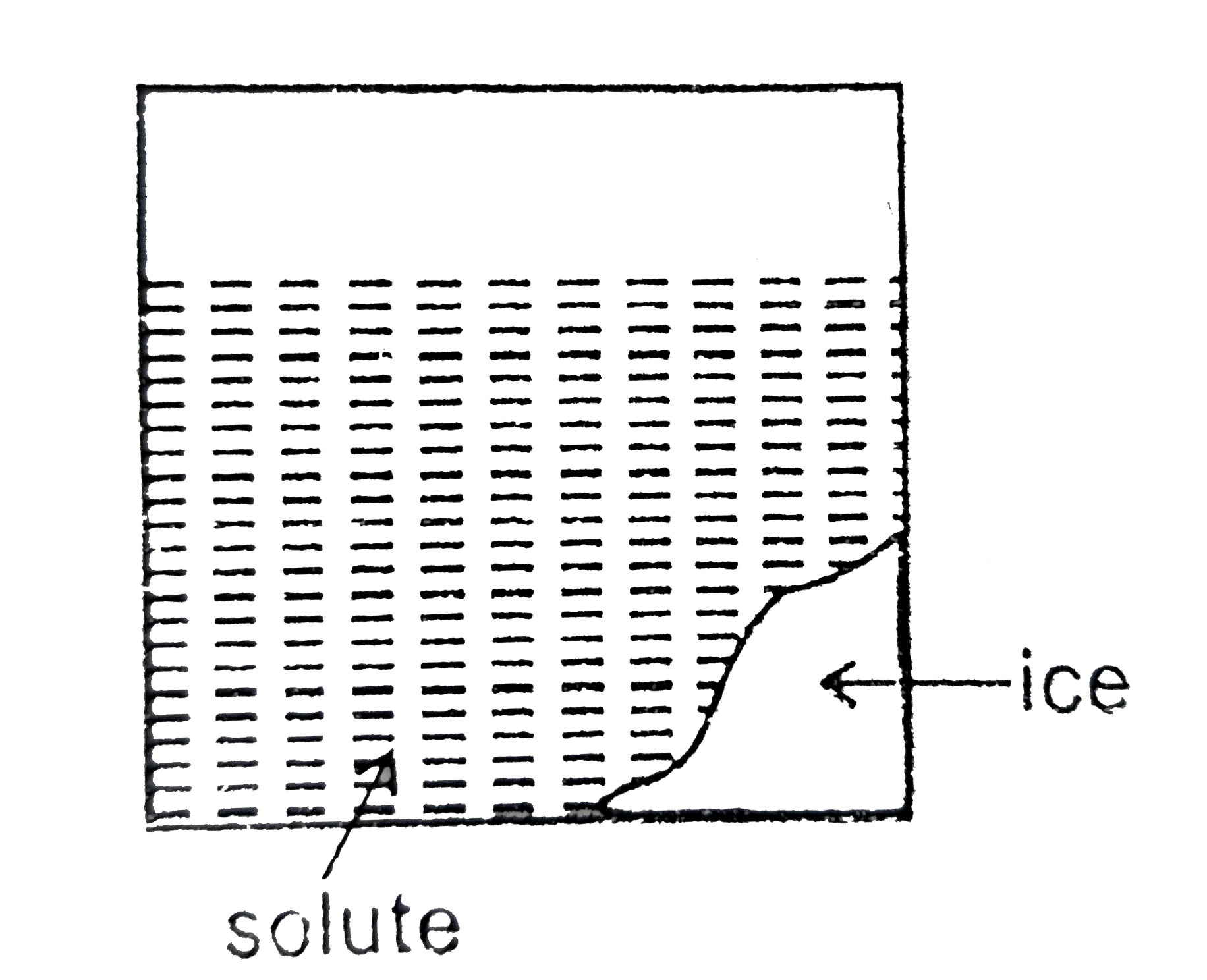
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1000gm H(2)O have 0.1 mole urea and its freezing point is-0.2^(@)C and...
Text Solution
|
- A solution of x moles of sucrose in 100grams of water freeze at -0.2^(...
Text Solution
|
- A solution of x moles of sucrose in 100 gram of water freezes at 00.2^...
Text Solution
|
- 1000gm H(2)O have 0.1 mole urea and its freezing point is- 0.2^(@)C an...
Text Solution
|
- 4.0g of substance A dissolved in 100g H(2)O depressed the freezing poi...
Text Solution
|
- जल का मोलल हिमांक स्थिरांक 1.86^(@)C//M है। 0.1 M सोडियम क्लोराइड विलय...
Text Solution
|
- 1000gm of sucrose solution in water is cooled to -0.5^(@)C . How much ...
Text Solution
|
- 0.1 mole of sugar is dissolved in 250 g of water. The freezing point o...
Text Solution
|
- 0.1 mole of sugar is dissolved in 250 g of water. The freezing point o...
Text Solution
|