Similar Questions
Explore conceptually related problems
Recommended Questions
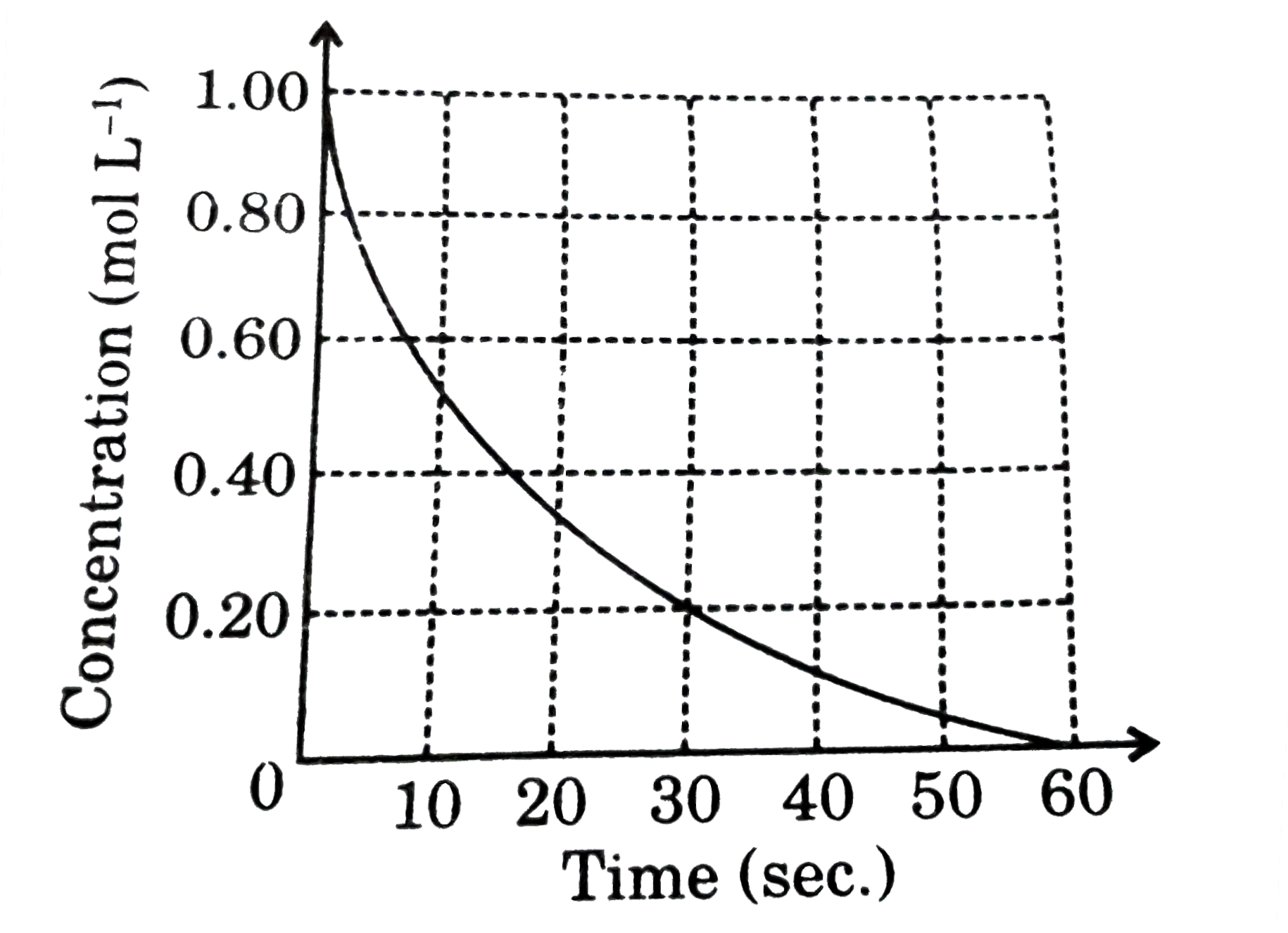
- According to the graph what is the rate of disappearance of the reacta...
Text Solution
|
- In a first order reaction, 75% of the reactants disappeared in 1.386 h...
Text Solution
|
- According to the graph what is the rate of disappearance of the reacta...
Text Solution
|
- द्रव्य अनुपाती क्रिया के नियमानुसार किसी रासायनिक अभिक्रिया की दर अभिक...
Text Solution
|
- A reaction is of second order with respect to a reactant. How is the r...
Text Solution
|
- Assertion. In any reaction, the rate of disappearance of a reactant is...
Text Solution
|
- What should be the rate of disappearance of B ?
Text Solution
|
- What should be the rate of formation of C, if the rate of disappearanc...
Text Solution
|
- A reaction is of second order with respect to a reactant. How is the r...
Text Solution
|