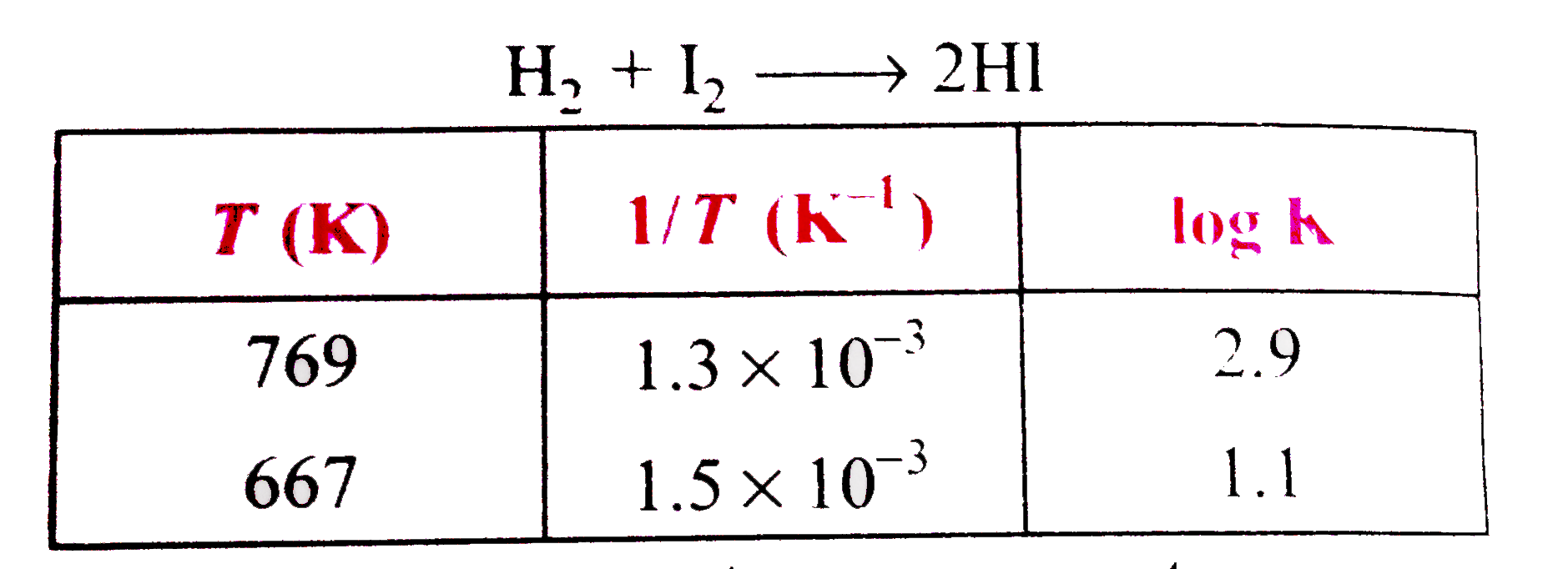Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data, the activation, energy for the reaction, (cal...
Text Solution
|
- If a reaction A+BrarrC is exothermic to the extent of 30 KJ mol^(-1), ...
Text Solution
|
- For the decomposition of HI the following logarithmic plot is shown : ...
Text Solution
|
- For the following data answer the questions : Reaction : A+B to P The ...
Text Solution
|
- From the following data, the activation, energy for the reaction, (cal...
Text Solution
|
- The activation energy of a reaction is 9.0 kcal//mol. The increase i...
Text Solution
|
- If a reaction A +B toC is exothermic to the extent of 30 kJ/mol and th...
Text Solution
|
- For a reaction A2 + B2 to 2AB , evaluate the energy of activation from...
Text Solution
|
- If a reaction , A+B to C , is exothermic to the extent of 30kJ//mol an...
Text Solution
|