Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate resonance energy for (1)/(6) mole of naphtalene if its heat ...
Text Solution
|
- Find the heat of hydrogenation of cyclohexene if the heat of hydrogena...
Text Solution
|
- Calculate resonance energy for (1)/(6) mole of naphtalene if its heat ...
Text Solution
|
- Heat of hydrogention of is 29 kcal mol^(-1) and that of is 56 "kcal" m...
Text Solution
|
- Heat of hydrogenation of cyclohexene to cyclohexane is -28.6 kcal/mol....
Text Solution
|
- Calculate the heat of formation of benzene from the following data, as...
Text Solution
|
- एसिटिलीन की दहन ऊष्मा 312 kcal हैं । यदि CO(2) की सम्भवन ऊष्मा त94.8 ...
Text Solution
|
- Given that : (i) heat of formation of water = -68.3 kcal (ii) heat of ...
Text Solution
|
- If the calculated and the experimental heats of combustion of benzene ...
Text Solution
|
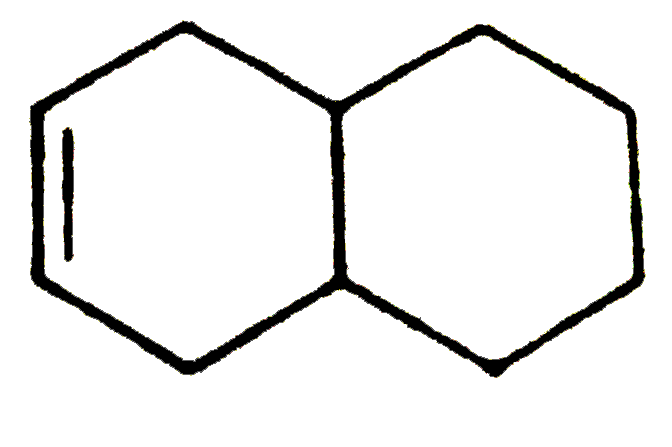 id 29 kcal.
id 29 kcal.