Similar Questions
Explore conceptually related problems
Recommended Questions
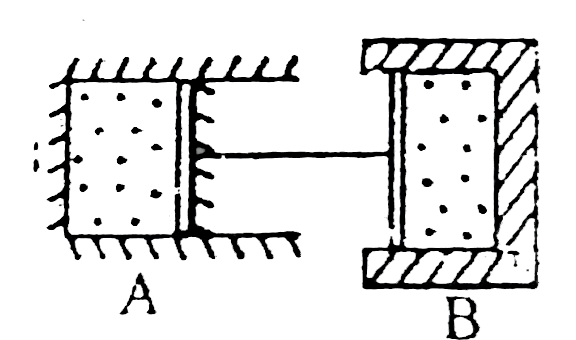
- Two cylinder A and B having piston conneted by massless rod (as shown ...
Text Solution
|
- Two cylinder A and B having piston conneted by massless rod (as shown ...
Text Solution
|
- Two cylinder A and B having piston conneted by massless rod (as shown ...
Text Solution
|
- Two cylinder A and B having piston conneted by massless rod (as shown ...
Text Solution
|
- Two cylinder A and B having piston conneted by massless rod (as shown ...
Text Solution
|
- Two cylinder A and B fitted with pistons contain equal number of moles...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- A cylinder with two ends is equipped with a moving piston. The gas on ...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|