Similar Questions
Explore conceptually related problems
Recommended Questions
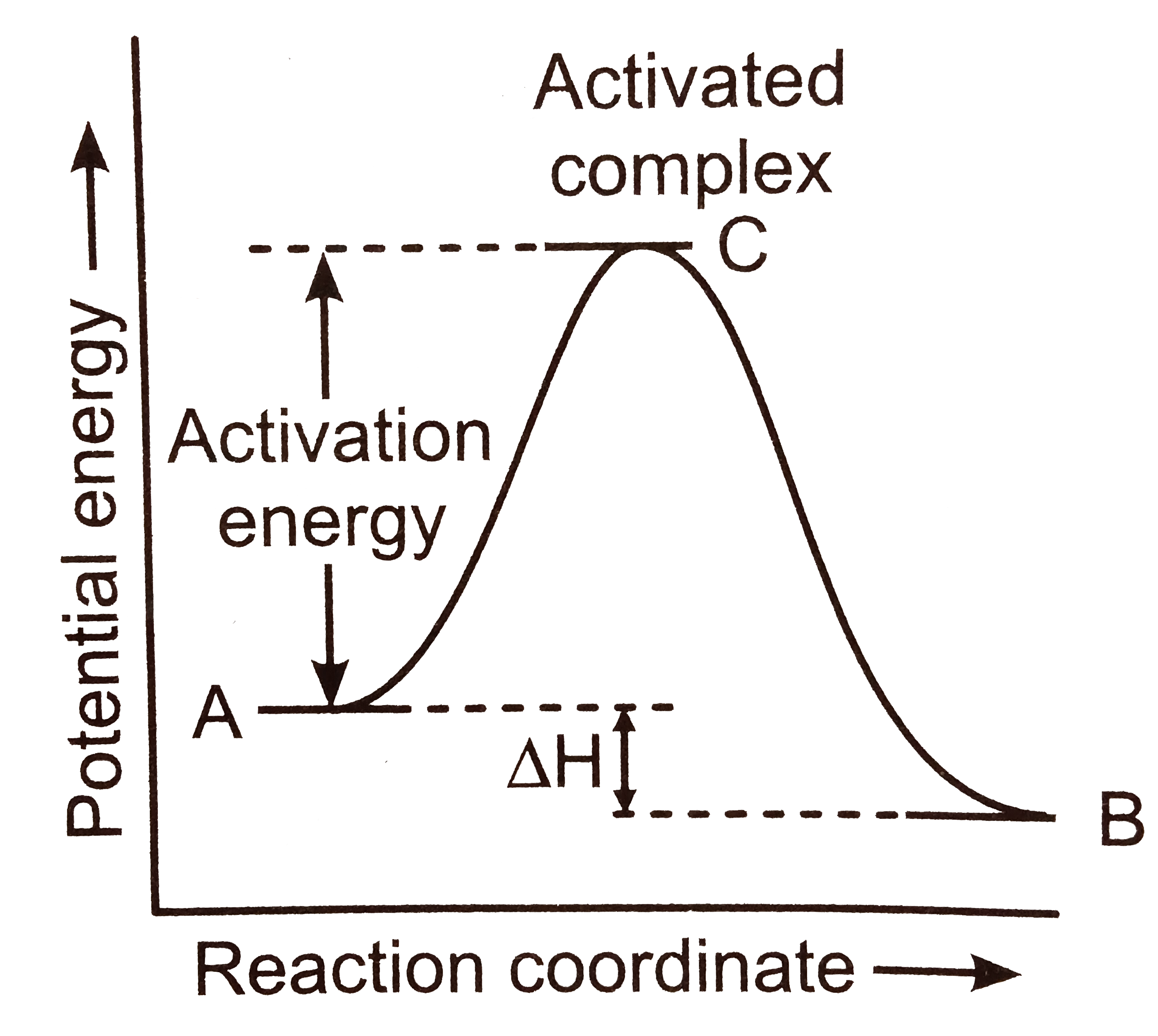
- The energy required to form the intermediate, called activated complex...
Text Solution
|
- The activation energy of reactant molecules in a reaction depends upon
Text Solution
|
- The energy required to form the intermediate, called activated complex...
Text Solution
|
- In the accompanied diagram E(R), E(P) and E(X) represents the energy o...
Text Solution
|
- Assertion: The enthalapy of reaction remains constant in the presence ...
Text Solution
|
- Potential energy of the products is than reactants in an endothermic ...
Text Solution
|
- The activation energy for the forward reaction of A+B hArr C+D is 50 k...
Text Solution
|
- Assertion (A) The enthalpy of reaction remains constant in the presenc...
Text Solution
|
- For a reaction XrarrY, heat of reaction is + a kJ, potential energy of...
Text Solution
|