Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
PRADEEP|Exercise Problem For Pactice|6 VideosS-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS)
PRADEEP|Exercise Assertion -Reason Type Question (Type 2)|19 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-SOLID STATE -Advanced problems
- A bcc lattic is made up of hollow spheres of X. spheres of soldid 'Y,'...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- The density of solid argon is 1.65g//mL at -233^(@)C . If the argon a...
Text Solution
|
- In the cubic crystal of CsCl (d = 3.97 g cm^(-3)), the eight corners a...
Text Solution
|
- An ionic compound AB has a rock salt structure with A :B = 1:1. the fo...
Text Solution
|
- An element crystallises in f.c.c. lattice having edge length 400 p m. ...
Text Solution
|
- In diamond lattice, all attice point and alternate tetrahedral voids a...
Text Solution
|
- A metallic crystal cystallizes into a lattice containing a sequence of...
Text Solution
|
- Calculate the distance between (111) planes in a crystal of calcium....
Text Solution
|
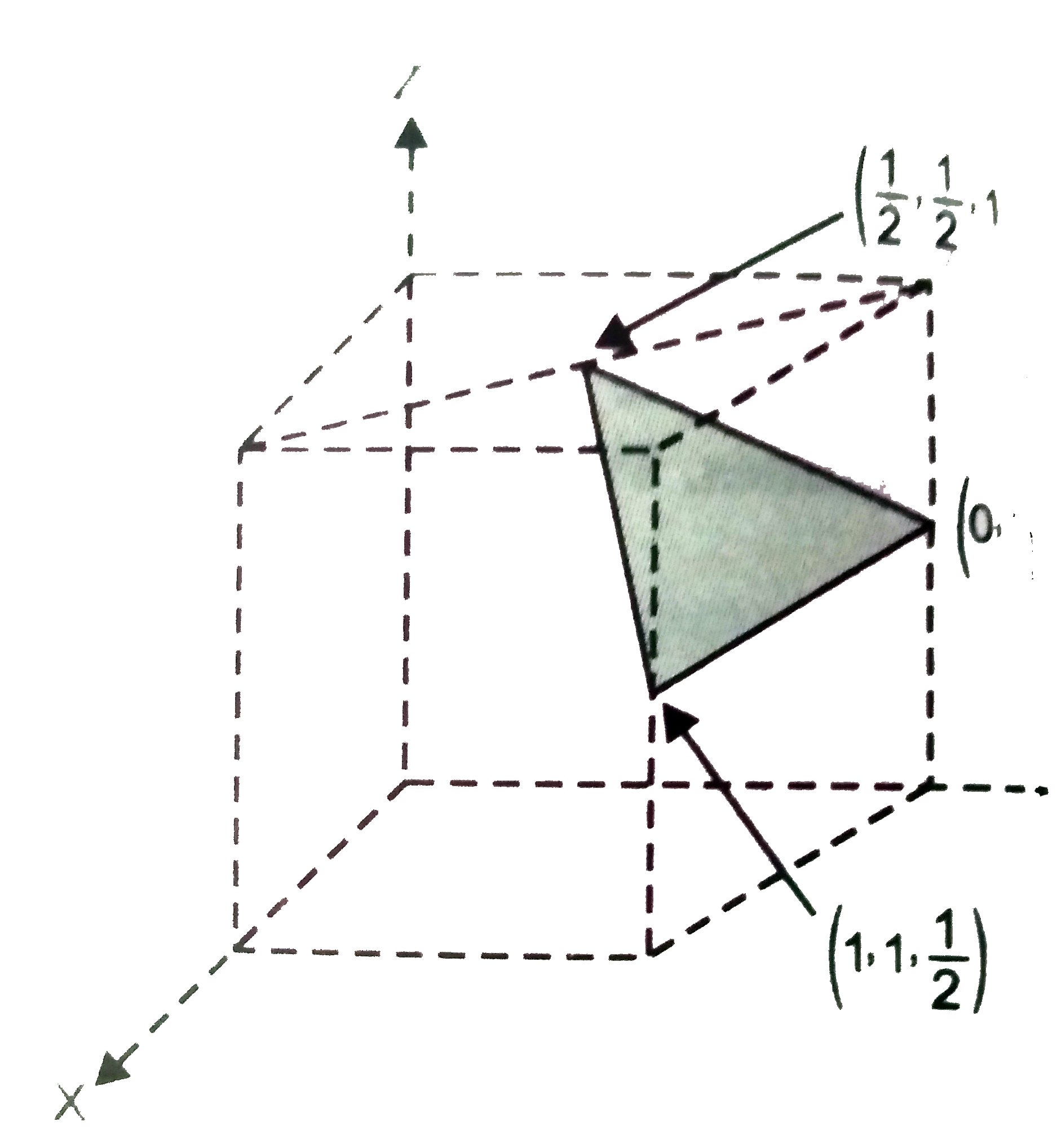
- Determine the miller indices of the shaded plane. Coordinates of the c...
Text Solution
|
- The coordinate of the three corners of a shaded face on a cubic unit c...
Text Solution
|
- The density of sodium chloride at 25^(@)C "is" 2.163 xx 10 ^(3) " kg...
Text Solution
|
- What fraction (n/N) of the lattice sites are vacant at 298 K for a cry...
Text Solution
|
- Metallic magnesium has a hexagonal close packed structure and a densit...
Text Solution
|
- Calculate the packing fraction and density of diamond if a = 3.57Å . ...
Text Solution
|
- Calculate the packing effeciency of a fcc crystal in which all the te...
Text Solution
|
- Using X -rays of wavelength 154.1 pm and staring from the glancing a...
Text Solution
|
- A reflaction from (111) planes of a cubic crystal was observed ad at a...
Text Solution
|
- When an electron in an excited state of Mo atom falls L to K -shell, a...
Text Solution
|