Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER: SOLID MATTER
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS (MATCHING)|3 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS (ASSERTION AND REASON)|5 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS (MULTIPLE CHOICE- II)|16 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)|12 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER: SOLID MATTER-NCERT EXEMPLAR PROBLEMS (SHORT ANSWER)
- why are liquids and gases categorised as fuids ?
Text Solution
|
- Why are solids incompressible ?
Text Solution
|
- Inspite of long range order in the arrangement of particles why...
Text Solution
|
- Why common salt (NaCl) sometimes appear yellow?
Text Solution
|
- why is Fe0(s) not formed in stoichiometric compostion ?
Text Solution
|
- why does white Zn0(s) becomes yellow upon heating ?
Text Solution
|
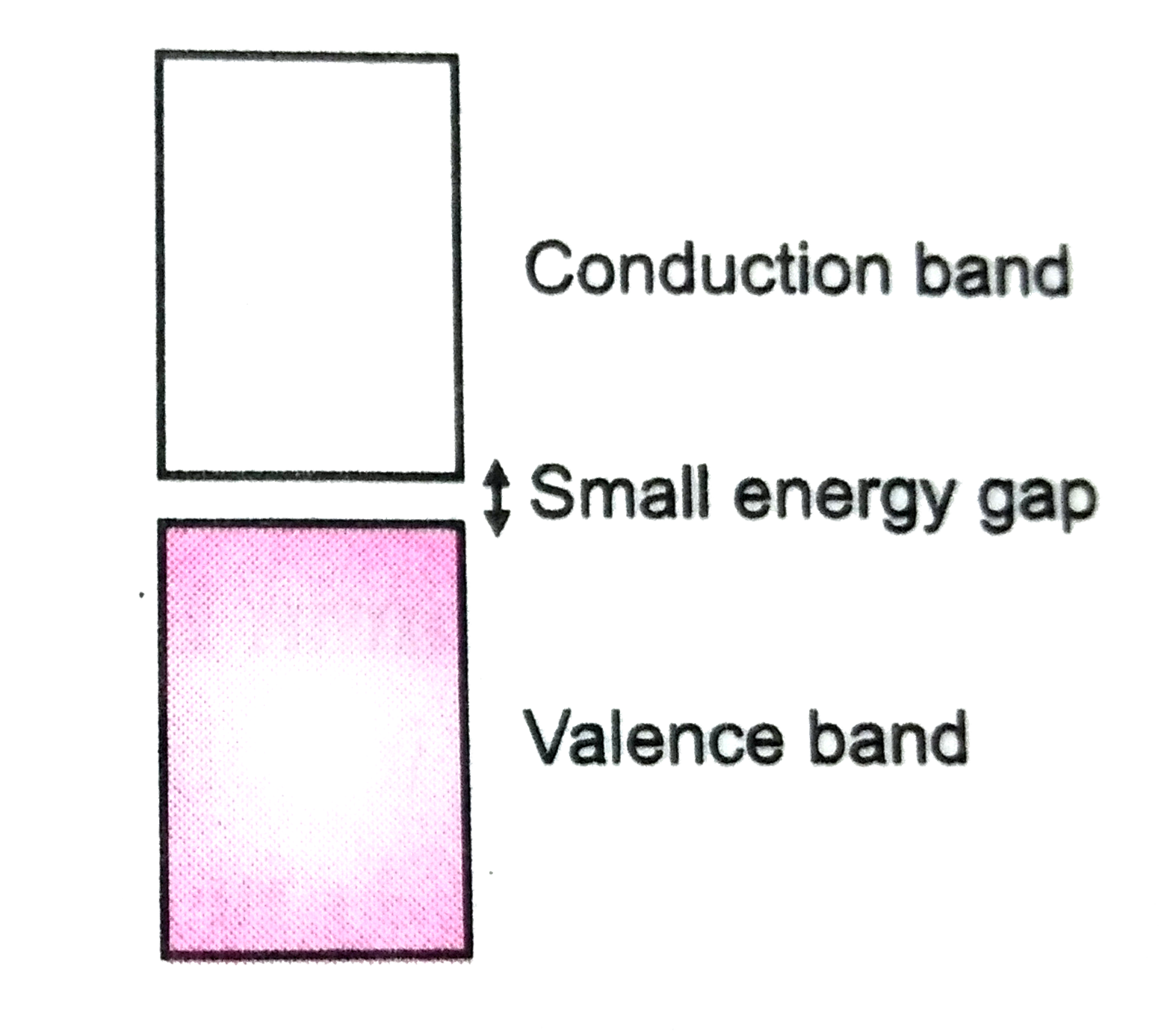
- Why does the electrical conductivity of semiconductors increase with r...
Text Solution
|
- Expalin why does conductivity of germainum crystals increase on ...
Text Solution
|
- In a compound, nitrogen atoms (N) make cubic close packed lattice and ...
Text Solution
|
- Under which situations can an amorphous substance change to cryst...
Text Solution
|