A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (II.MULTIPLE CHOICE)|10 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (III.MULTIPLE CHOICE)|11 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise ANALYTICAL (PROBLEMS )|11 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)|12 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER: SOLID MATTER-COMPETITION FOCUS (I.MULTIPLE CHOICE)
- A given metal crystalline out with a cubic structure having edge leng...
Text Solution
|
- The edge length of a face-centred cubic unit cell is 508 pm. If the ra...
Text Solution
|
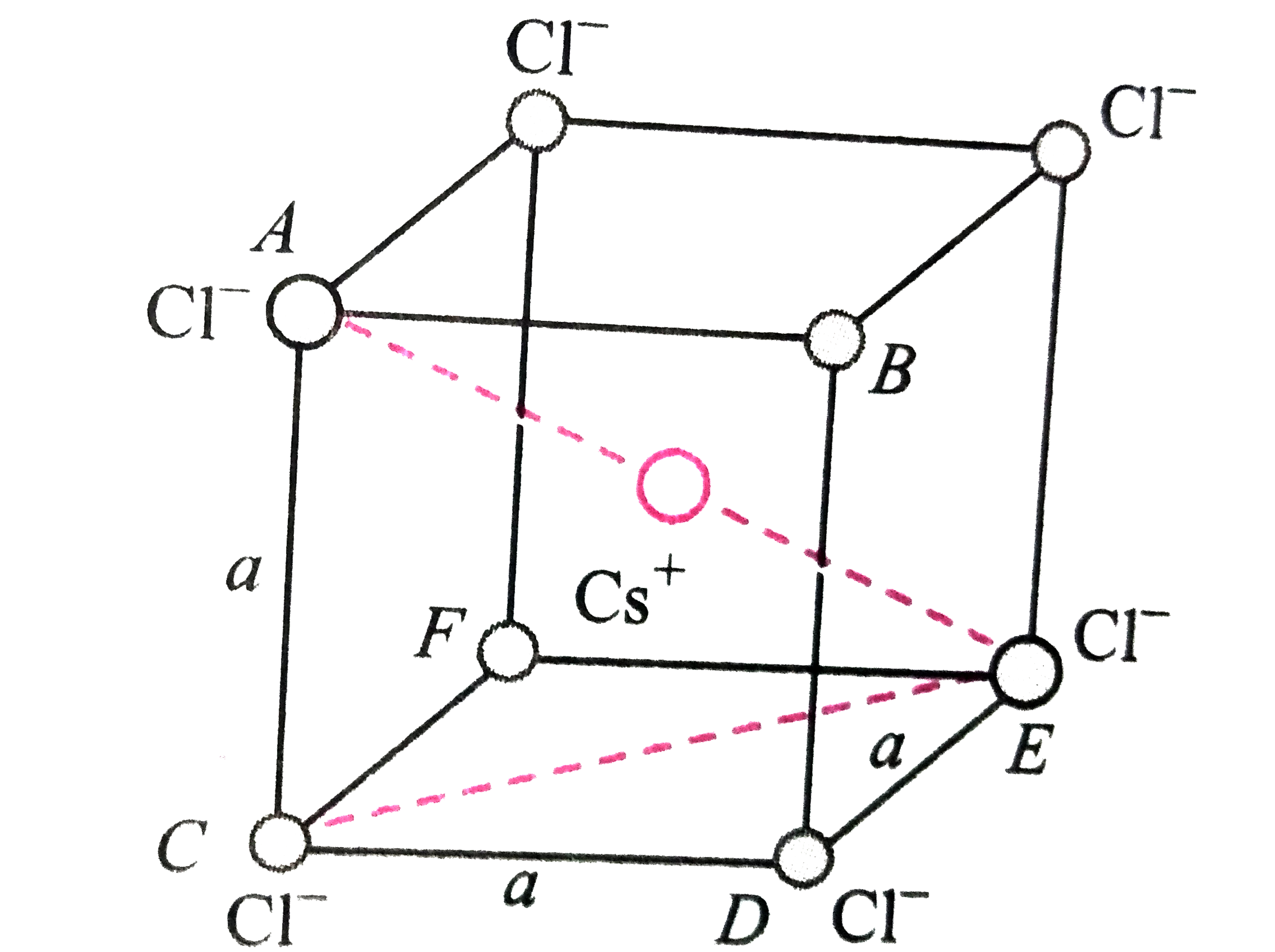
- CsClcrystallizes in body centred cubic lattice. If 'a' is its edge len...
Text Solution
|
- If a is the length of the side of a cube, the distance between the bod...
Text Solution
|
- The edge length of a cube is 400 pm .its body diagonal would be
Text Solution
|
- If 'a' stands for the edge length of the cubic systems: simple cubic,b...
Text Solution
|
- A metal has a fcc lattice.The edge length of the unit cell is 404 pm ,...
Text Solution
|
- The number of atoms is 100 g of a fcc crystal with density = 10.0 g//...
Text Solution
|
- Ice crystallises in hexagonal lattice having volume of unit cell is 13...
Text Solution
|
- if the edge length of a NaH unit cell is 488 pm, what is the length of...
Text Solution
|
- Lithium has a bcc structure .Its density is 530 kg m^(-3) and its atom...
Text Solution
|
- Iron exhibits bcc structure at roomj temperature. Above 9000^(@)C, it ...
Text Solution
|
- The correct statement regarding defects in crystalling solids.
Text Solution
|
- If NaCl is doped with 10^(-4)mol%of SrCl(2) the concentration of cati...
Text Solution
|
- The crystal with metal deficiency defect is:
Text Solution
|
- Experimentally it was found that a metal oxide has formula M(0.98) O. ...
Text Solution
|
- Which type of 'defect' has the pressence of cations in the interstitia...
Text Solution
|
- The substances,Which are repelled by a magnet,are termed as
Text Solution
|
- Which of the following metal oxides is anti-ferromagnetic in nature?
Text Solution
|
- The energy gaps (E(g)) between valence band and conduction band for di...
Text Solution
|