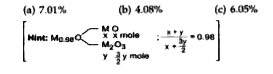A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (II.MULTIPLE CHOICE)|10 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (III.MULTIPLE CHOICE)|11 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise ANALYTICAL (PROBLEMS )|11 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)|12 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER: SOLID MATTER-COMPETITION FOCUS (I.MULTIPLE CHOICE)
- if the edge length of a NaH unit cell is 488 pm, what is the length of...
Text Solution
|
- Lithium has a bcc structure .Its density is 530 kg m^(-3) and its atom...
Text Solution
|
- Iron exhibits bcc structure at roomj temperature. Above 9000^(@)C, it ...
Text Solution
|
- The correct statement regarding defects in crystalling solids.
Text Solution
|
- If NaCl is doped with 10^(-4)mol%of SrCl(2) the concentration of cati...
Text Solution
|
- The crystal with metal deficiency defect is:
Text Solution
|
- Experimentally it was found that a metal oxide has formula M(0.98) O. ...
Text Solution
|
- Which type of 'defect' has the pressence of cations in the interstitia...
Text Solution
|
- The substances,Which are repelled by a magnet,are termed as
Text Solution
|
- Which of the following metal oxides is anti-ferromagnetic in nature?
Text Solution
|
- The energy gaps (E(g)) between valence band and conduction band for di...
Text Solution
|
- Which of the following compounds is metallic and ferromagnetic ?
Text Solution
|
- For which crystal anion-anion contact is valid ?
Text Solution
|
- Each rubidium halide crystallising in the NaCl-type lattice has a unit...
Text Solution
|
- For a solid with the adjoining structure, the coordination number of t...
Text Solution
|
- If the positions of Na^(+) and Cl^(-) are interchanged in NaCl, havin...
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- KCl crystallises in the same type of lattices as does NaCl. Given tha...
Text Solution
|
- Which has no rotaition of symmetry ?
Text Solution
|
- Which is the incorrect statement ?
Text Solution
|