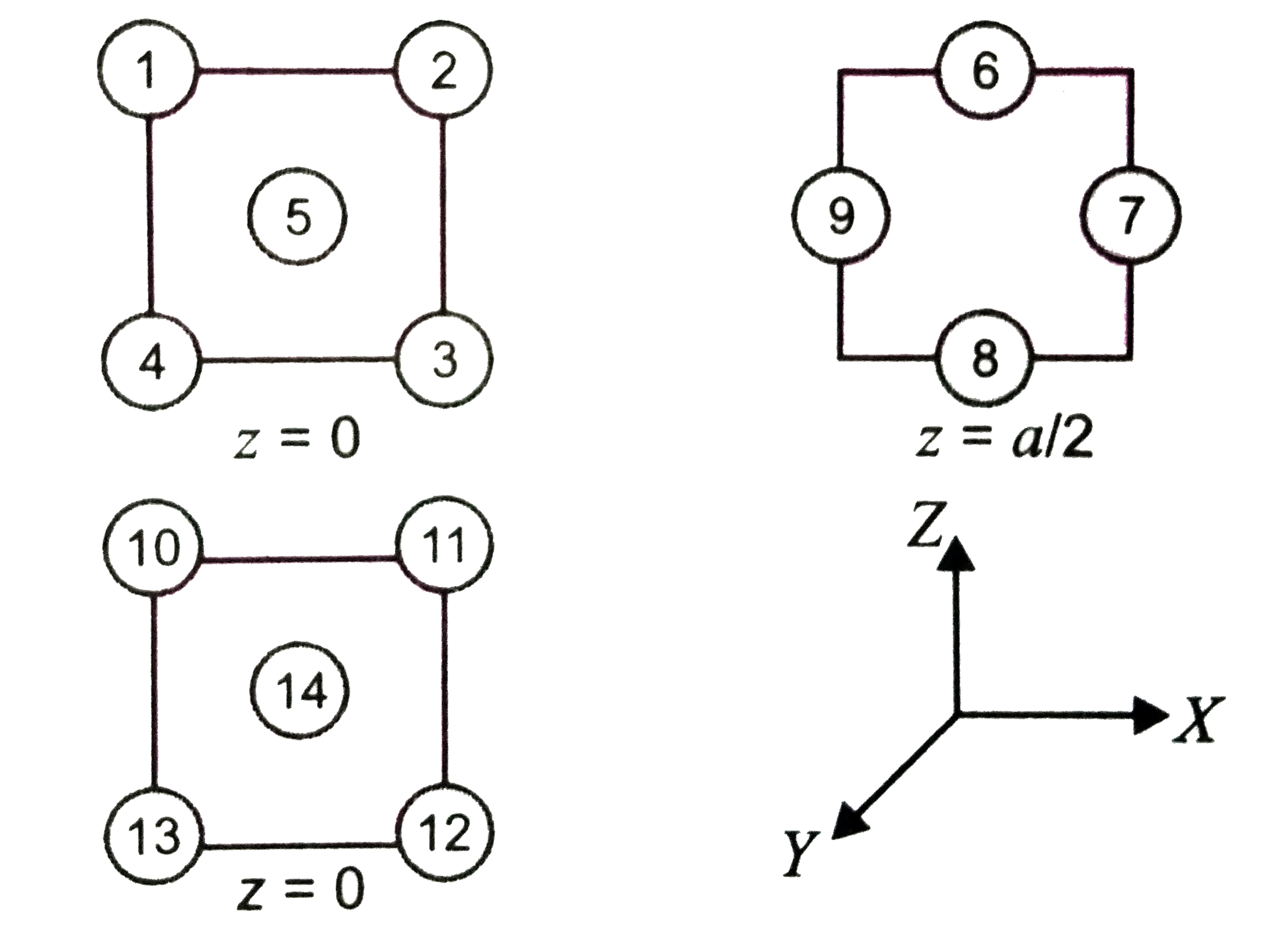
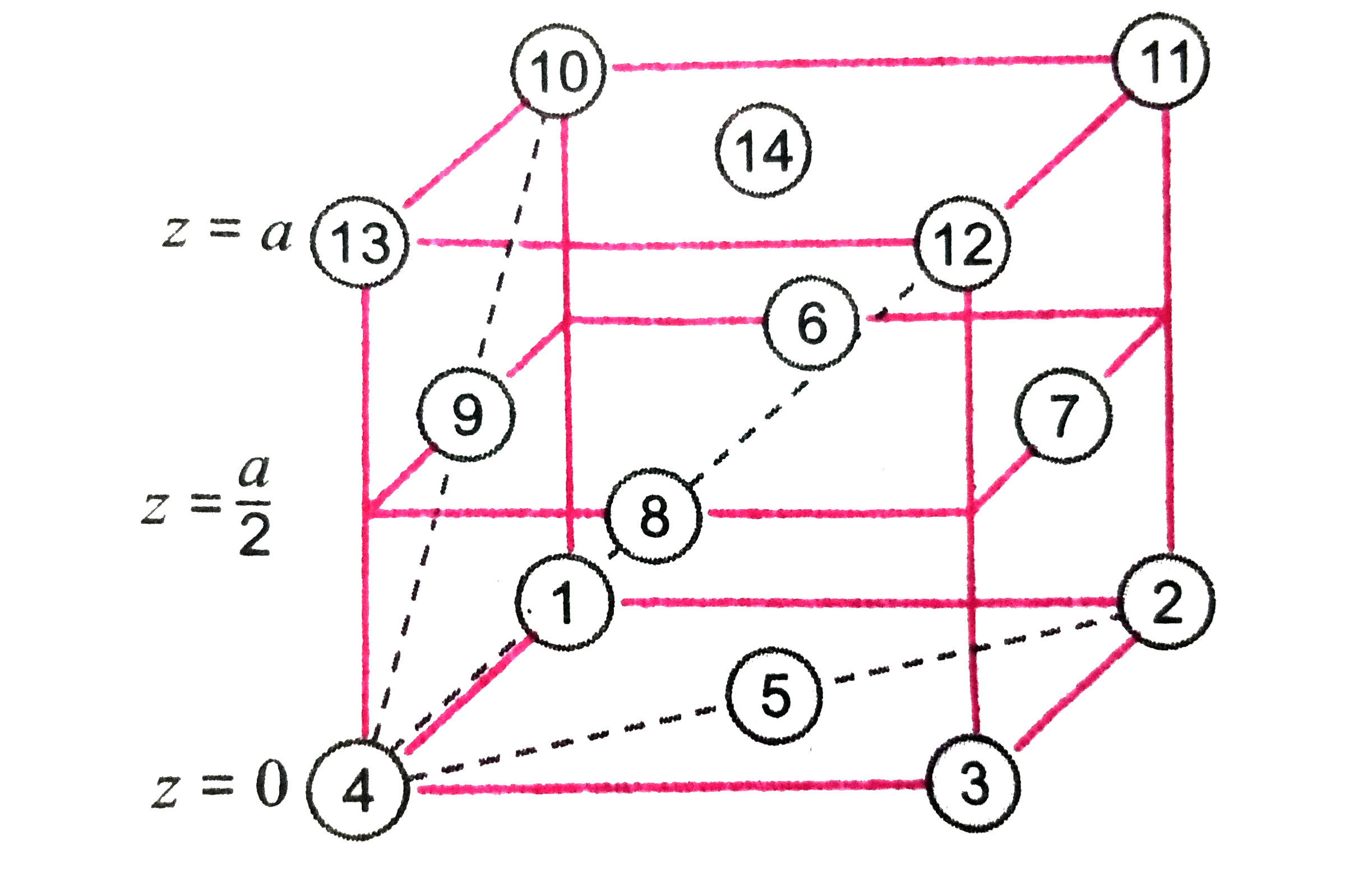
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (III.MULTIPLE CHOICE)|11 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (MATCHING)|2 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (I.MULTIPLE CHOICE)|52 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)|12 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER: SOLID MATTER-COMPETITION FOCUS (II.MULTIPLE CHOICE)
- Which of the following statements are not true ?
Text Solution
|
- Which of the following are not true about hexagonal close packing ?
Text Solution
|
- Which of the following are true ?
Text Solution
|
- Crystal systems in which no two axial lengths are equal are
Text Solution
|
- A metal has cubic close packed (ccp) arrangement , the layer sequence ...
Text Solution
|
- The density of KBr is 2.75 g cm^(-3). The length of the unit cell is 6...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- The correct statement(s) regarding defects in solids is (are)
Text Solution
|
- With respect to graphite and diamond, which of the following statement...
Text Solution
|
- The correct statement (s) for cubic close packed (ccp) three dimension...
Text Solution
|