A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (MATCHING)|2 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (INTEGER)|9 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (II.MULTIPLE CHOICE)|10 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)|12 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
PRADEEP-STATES OF MATTER: SOLID MATTER-COMPETITION FOCUS (III.MULTIPLE CHOICE)
- By X-ray studies, the packing atoms, in a crystal of gold is found to ...
Text Solution
|
- By X-ray studies, the packing atoms, in a crystal of gold is found to ...
Text Solution
|
- By X-ray studies, the packing of atoms in a crystal of gold is found t...
Text Solution
|
- By X-ray studies, the packing atoms, in a crystal of gold is found to ...
Text Solution
|
- By X-ray studies, the packing atoms, in a crystal of gold is found to ...
Text Solution
|
- No crystal is found to be prefect at room temperature. The defects pre...
Text Solution
|
- No crystal is found to be prefect at room temperature. The defects pre...
Text Solution
|
- No crystal is found to be prefect at room temperature . The defects pr...
Text Solution
|
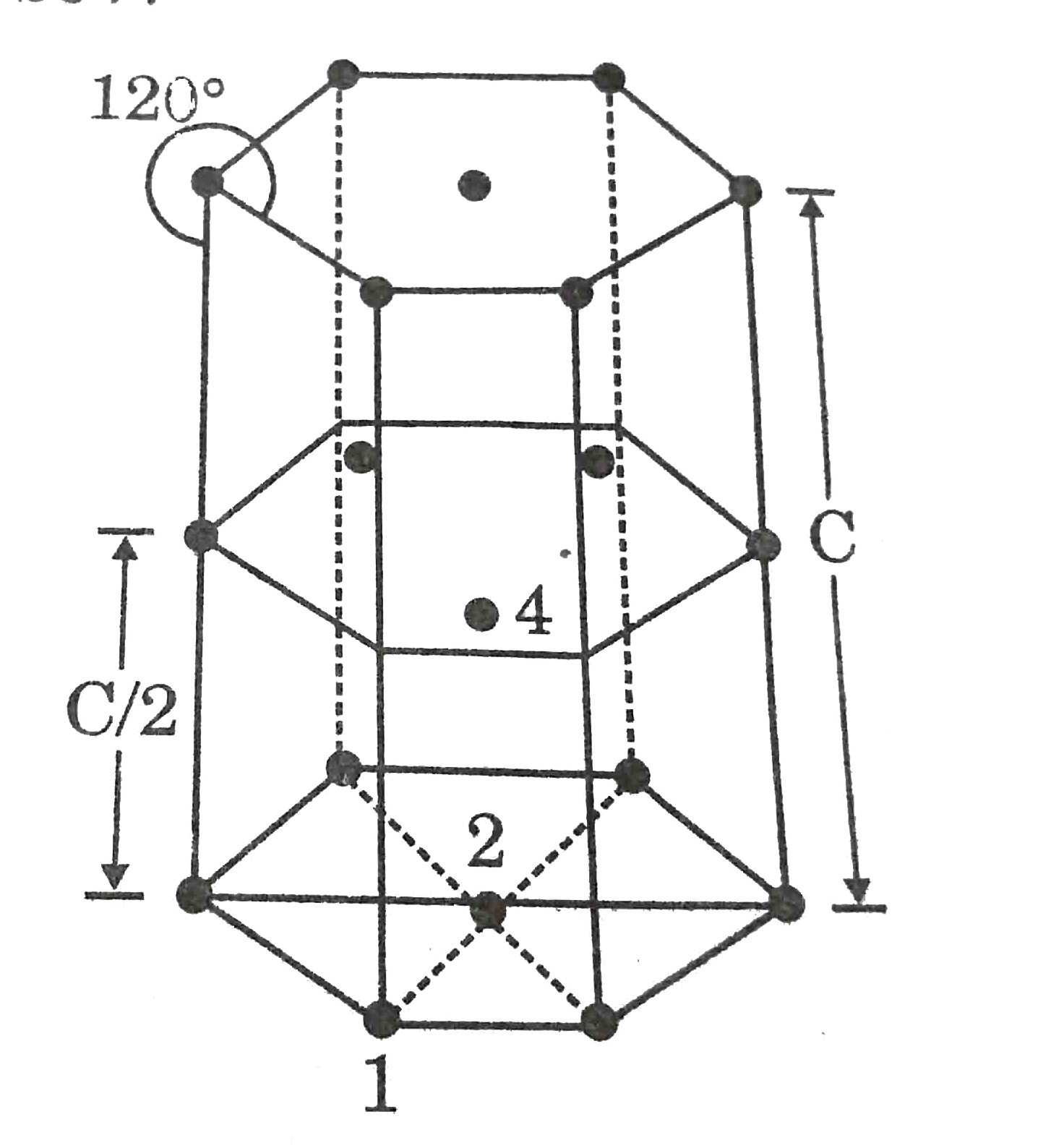
- In hexagonal system of crystals, a frequently encountered arrangement ...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In hexogonal systems of crystals, a frequently encountered arrangement...
Text Solution
|