Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- The standard enthalpy of formation of octane (C(8)H(18)) is -250kJ //m...
Text Solution
|
- Calculate the enthalpy of formation of acetic acid (CH(3)COOH) if its ...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- The enthalpy change of formation of CO(2)(g) is -393 kJ mol^(-1) and t...
Text Solution
|
- From the data given below at 298 K for the reaction : CH(4)(g) + 2O(2)...
Text Solution
|
- Calculate the value of enthalpy of combustion of cyclopropane at 25^(@...
Text Solution
|
- The enthalpy of combustion of methane, graphite and dihydrogen at 298K...
Text Solution
|
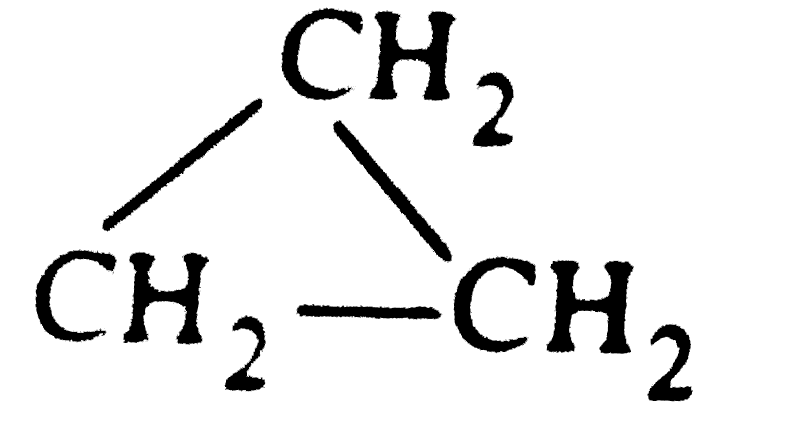 ` rarrCH_(3)- CH=CH_(2), DeltaH = -33.0 kJ `
` rarrCH_(3)- CH=CH_(2), DeltaH = -33.0 kJ `