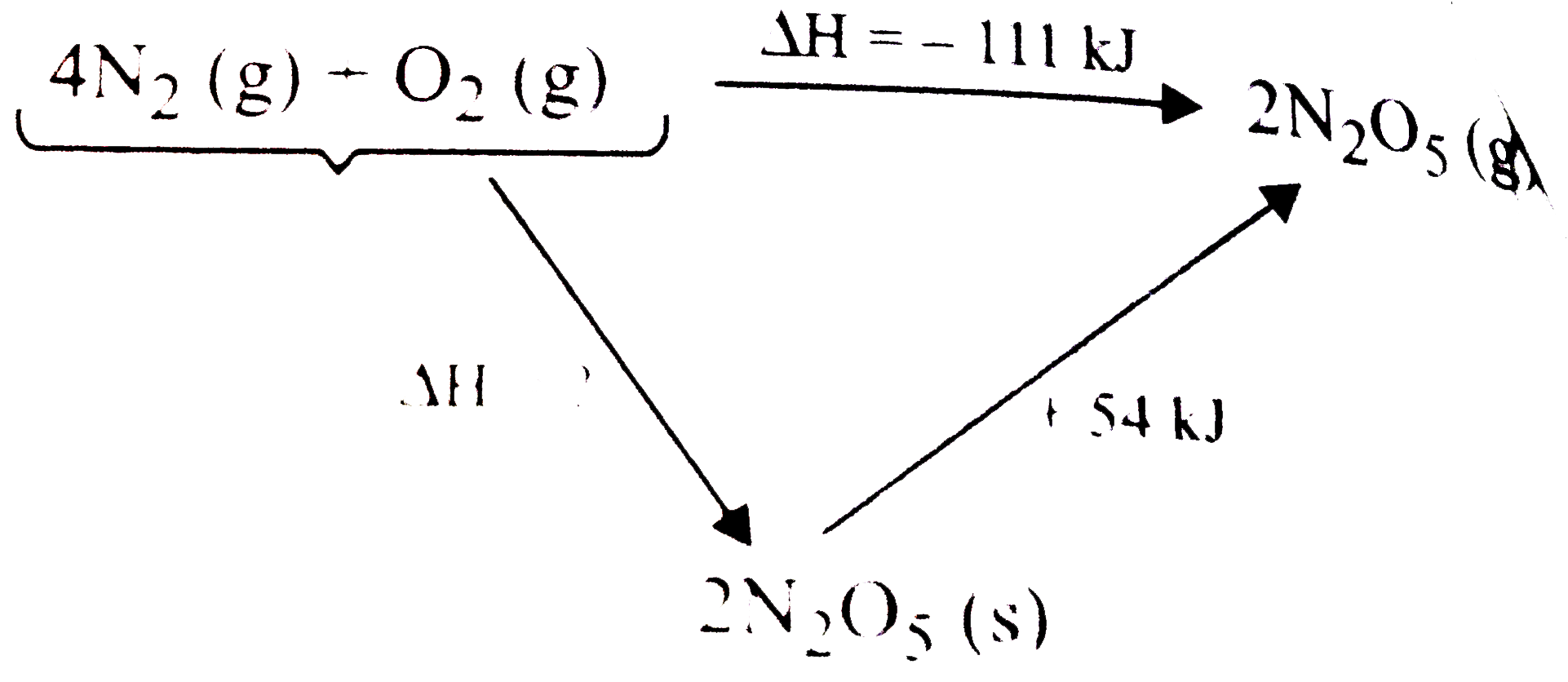
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Comprehension 1|1 VideosTHERMODYNAMICS
PRADEEP|Exercise Comprehension 2|2 VideosTHERMODYNAMICS
PRADEEP|Exercise SHORT ANSWER QUESTIONS|65 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-COMPETITION FOCUS (JEE ( main andAdvanced ) /Medical Entrance Special)
- Enthalpy of CH(4)+(1)/(2)O(2)rarrCH(3)OH is negative. If enthalpy of...
Text Solution
|
- The standard heats of formation of NO(2)(g) and N(2)O(4)(g) are 8.0 an...
Text Solution
|
- Consider the reaction, 4NO(2)(g)+O(2)(g)rarr2N(2)O(5)(g),Delta(r )H=...
Text Solution
|
- Consider the following processes :- {:(,DeltaH(kJ//mol)),((1)/(2)A r...
Text Solution
|
- The standard enthalpies fo formation of CO(2) (g), H(2) O (1), and glu...
Text Solution
|
- The heats of combustion of carbon and carbon monoxide are -393.5and-28...
Text Solution
|
- Given C(("graphite"))+O(2)(g)toCO(2)(g), Delta(r)H^(0)=-393.5kJ" "mo...
Text Solution
|
- The bond energy of an O -H bond is 109 K. cal "mole"^(-1). When a mole...
Text Solution
|
- If the bond dissociation energies of XY,X(2) and Y(2)( all diatomic mo...
Text Solution
|
- For the reaction: 2H(2)(g) +O(2)(g) rarr 2H(2)O(g), DeltaH =- 571 kJ...
Text Solution
|
- Formation of ozone takes place as O(2)(g) + O(g) rarr O(3)(g) , DeltaH...
Text Solution
|
- Calculate the standard enthalpy change (in kJ "mol"^(-1)) for the reac...
Text Solution
|
- Enthalpy change for the reaction, 4H((g))rarr 2H(2(g)) is -869.6 kJ ...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ "mol"...
Text Solution
|
- The bond dissociation energies ofX(2),Y(2)andXY are in the ratio of1:0...
Text Solution
|
- For the reaction 2H(g)rarr H(2)(g), the sign of DeltaH and DeltaS resp...
Text Solution
|
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- If water kept in an insultated vessel at - 10^(@)C suddenly freezes, ...
Text Solution
|
- The direct conversion of A to B is difficult. Hence it is carried out ...
Text Solution
|
- Given an idealgas is expanded adiabatically and irreversibley form vol...
Text Solution
|