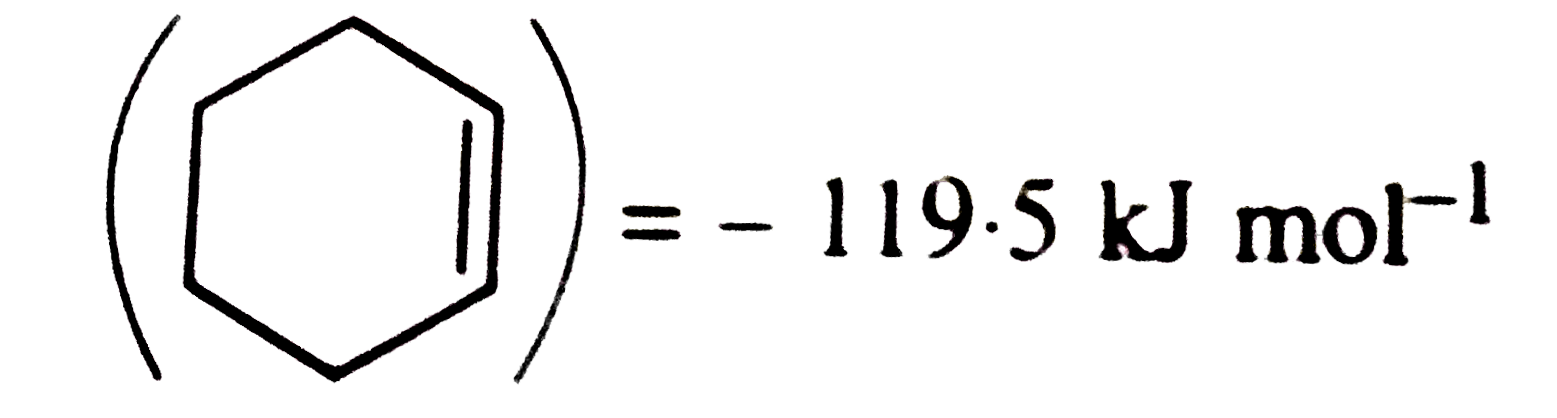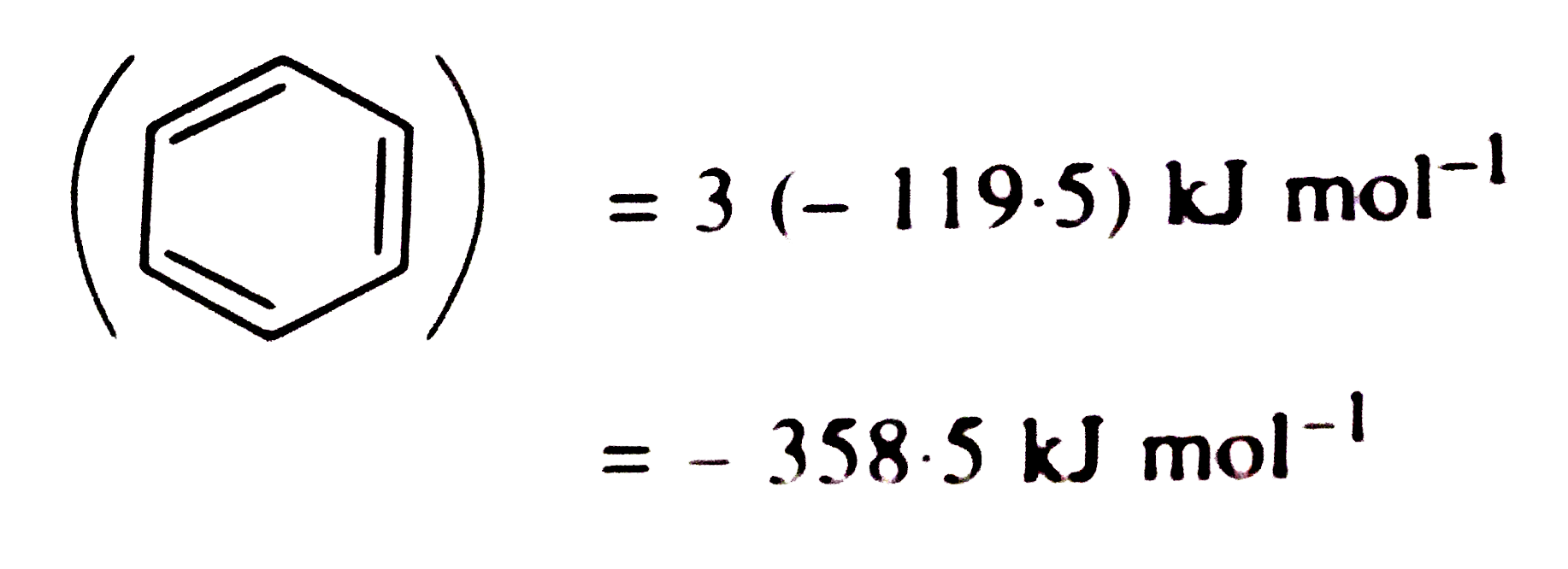A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Comprehension 1|1 VideosTHERMODYNAMICS
PRADEEP|Exercise Comprehension 2|2 VideosTHERMODYNAMICS
PRADEEP|Exercise SHORT ANSWER QUESTIONS|65 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-COMPETITION FOCUS (JEE ( main andAdvanced ) /Medical Entrance Special)
- Efficiency of Carnot engine is 100% if
Text Solution
|
- An ideal gas is allowed to expand both reversibly and irreversibly in ...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5KJ . If resonanc...
Text Solution
|
- Water is supercooled to - 4^(@)C. The enthalpy (H) is
Text Solution
|
- Enthalpy is equal to
Text Solution
|
- Consider the following liquid-vapour equilibrium. "Liquid" hArr "Vap...
Text Solution
|
- The standard state Gibbs free energies of formation of ) C(graphite an...
Text Solution
|
- For the adiabatic expansion of an ideal gas:
Text Solution
|
- In which of the following entropy increases?
Text Solution
|
- The criteria for spontaneity of a process is/are
Text Solution
|
- Which of the following statement are not correct ?
Text Solution
|
- Which of the following relationship are correct?
Text Solution
|
- Which of the following reactions is an endothermic reaction?
Text Solution
|
- Among the following , the state funcation (s) is (are)
Text Solution
|
- For an ideal gas,consider only P-V work in going from an initial state...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- An ideal gas in a thermally insulated vesselat internal pressure =P(1)...
Text Solution
|
- For a reaction taking place in a container in equilibrium with its sur...
Text Solution
|
- An ideal gas is expand from (p(1),V(1),T(1)) to (p(2),V(2),T(2)) under...
Text Solution
|
- A reversible cyclic process for an ideal gas is shown below. Here P,V...
Text Solution
|