Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
PRADEEP|Exercise NCERT Questions and Exercises with Answers (NCERT INTEXT SOLVED QUESTIONS)|40 VideosHYDROGEN
PRADEEP|Exercise NCERT Questions and Exercises with Answers (NCERT Exercises)|1 VideosHYDROGEN
PRADEEP|Exercise CONCEPTUAL QUESTIONS (Dihydrogen)|7 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS|31 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions|25 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROGEN -CONCEPTUAL QUESTIONS (Water)
- Name one reaction in which water acts (i) as an oxidising agent and (i...
Text Solution
|
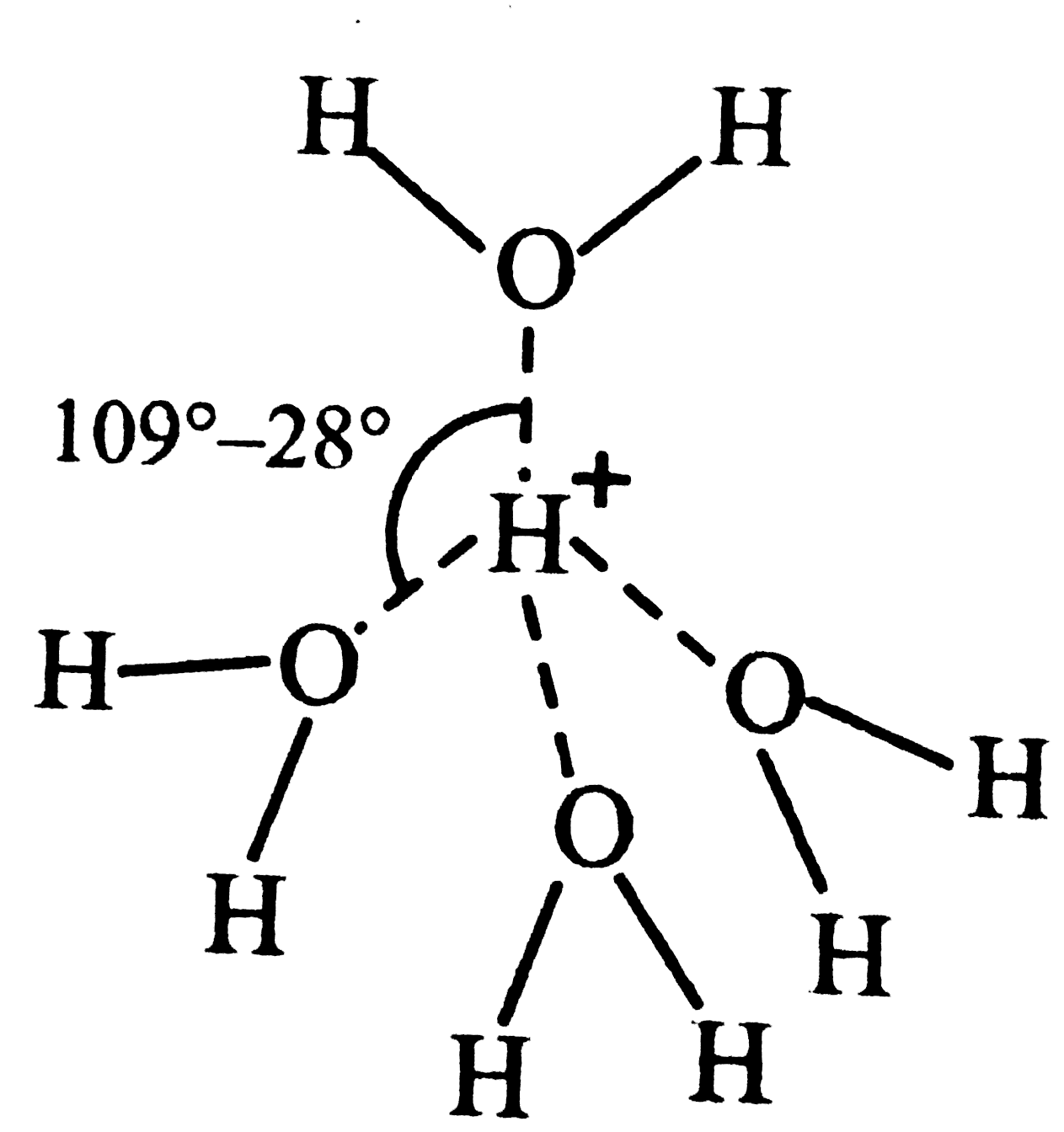
- a. What does [H(9)O(4)]^(o+) stand for ? Draw its structures. b. Can...
Text Solution
|
- An aqueous compound of an inorganic compound (X) shows the following r...
Text Solution
|
- Why is hydrated barium peroxide used in the preparation of hydrogen pe...
Text Solution
|
- A white solid is either Na(2)O or Na(2)O(2). A piece of red litmus pap...
Text Solution
|
- Status coated with white lead on long exposure to atmosphere turn blac...
Text Solution
|
- A mixture of hydrazine and H(2)O(2) with Cu(II) catalyst is used as a ...
Text Solution
|