Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Curiosity Questions|7 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Problem For Practice|27 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-Competition Focus (Jee (Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions
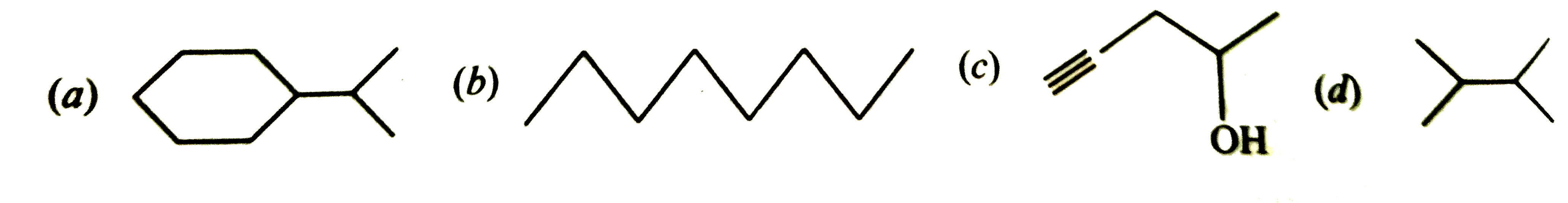
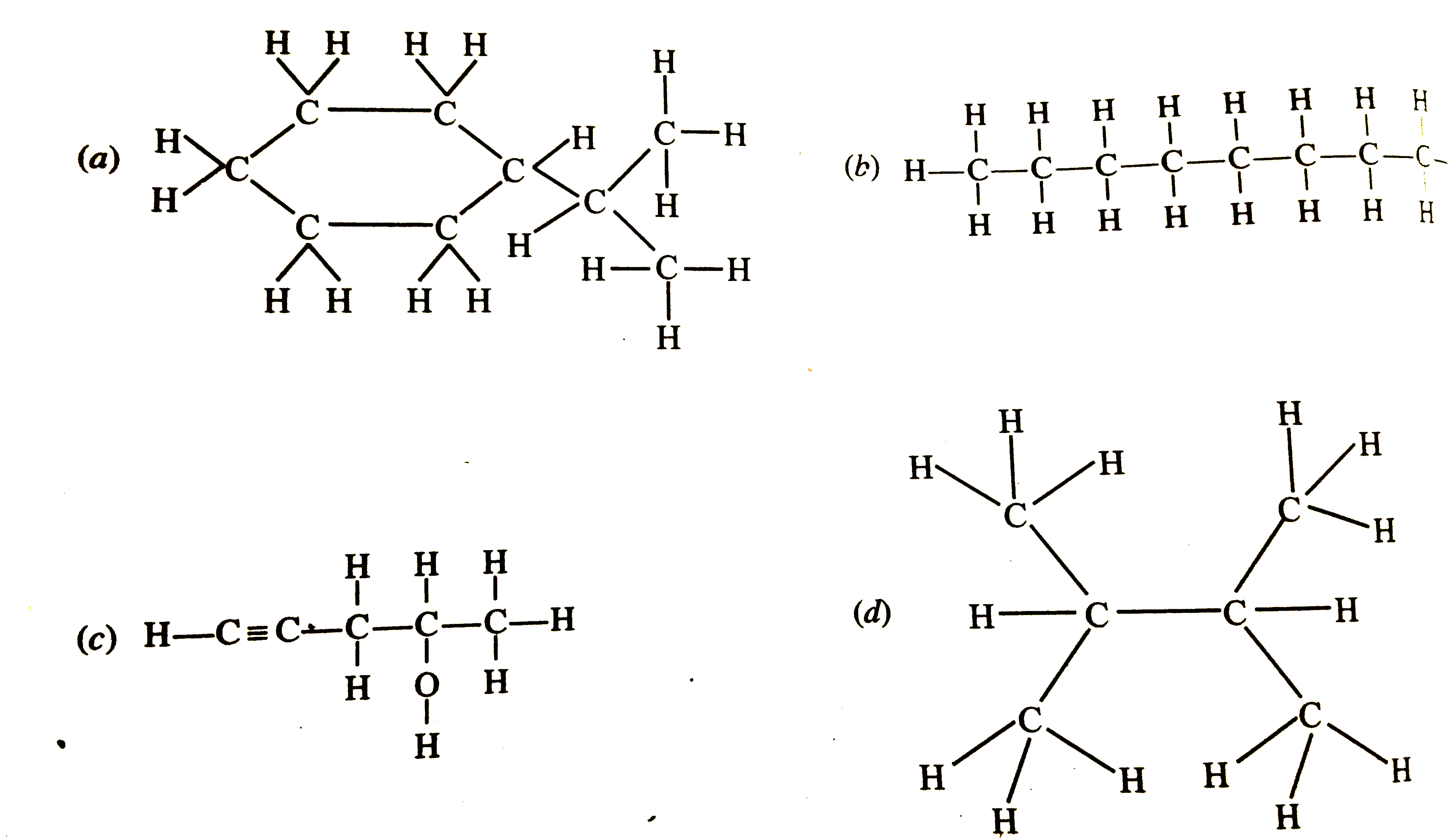
- Expand each of the following bond-line formulae to show all the atoms ...
Text Solution
|
- Statement-1 . The IUPAC name of the compound {:(CH(2)=CH-CH=CH-CH-C-...
Text Solution
|
- Assertion: CH(3)CHO and CH(2)=CHOH are resonance structures. Reason:...
Text Solution
|
- Statement-1. Simple carbanions are usually pyramidal but allyl carbani...
Text Solution
|
- Assertion: tert-Butyl carbanion is less stable than methyl carbanion. ...
Text Solution
|
- Assertion: Free redicals are always planer. Reason: They can achieve...
Text Solution
|
- Assertion: Nitrenes cannot be isolated. Reason: Nitrene are the nitr...
Text Solution
|
- Statement-1. Beilstein test can be used to detect fluorine in the orga...
Text Solution
|
- Statement-1. Lassaigne's extract is boiled with dil. HNO(3) before tes...
Text Solution
|
- Assertion : Butane and 2-methyl butane are chain isomers. Reason : B...
Text Solution
|
- Assertion :But-1-ene2-Methylprop-1-ene are position isomers. Reason Po...
Text Solution
|
- Assertion : All the carbon atoms of but-2-ene lie in one plane. Reas...
Text Solution
|
- Assertion : In CH(2)=C=CH(2), all the carbon atoms are sp^(2)-hybridiz...
Text Solution
|
- Assertion : A free radical is paramagnetic species. Reason : A free ...
Text Solution
|
- (A) Tertiary carbocations are generally formed more easily than primar...
Text Solution
|
- Assertion: Alkyl carbonaions like ammonia have pyramidal shape. Reas...
Text Solution
|
- (A) Tertiary butyl carbonion is less stable than methyl carbanion. (...
Text Solution
|
- (A) Allyl free radical is more stable than simple alkyl free radical. ...
Text Solution
|
- Assertion (A) o-and p-nitrophenol can be separated by steam distillati...
Text Solution
|
- Assertion : Essential oils are purified by steam distillation. Reaso...
Text Solution
|
- (A) Oils are purified by steam distillation. (R) The compounds which...
Text Solution
|