Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - I)|15 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - II)|7 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Conceptual Questions (IV. Purification and Separation of Organic Compounds)|11 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-NCERT Questions and Exercises With Answers
- What are hybridisation states of each carbon atom in the following c...
Text Solution
|
- Indicate the sigma-and -bonds in the following molecules : C(6)H(6)...
Text Solution
|
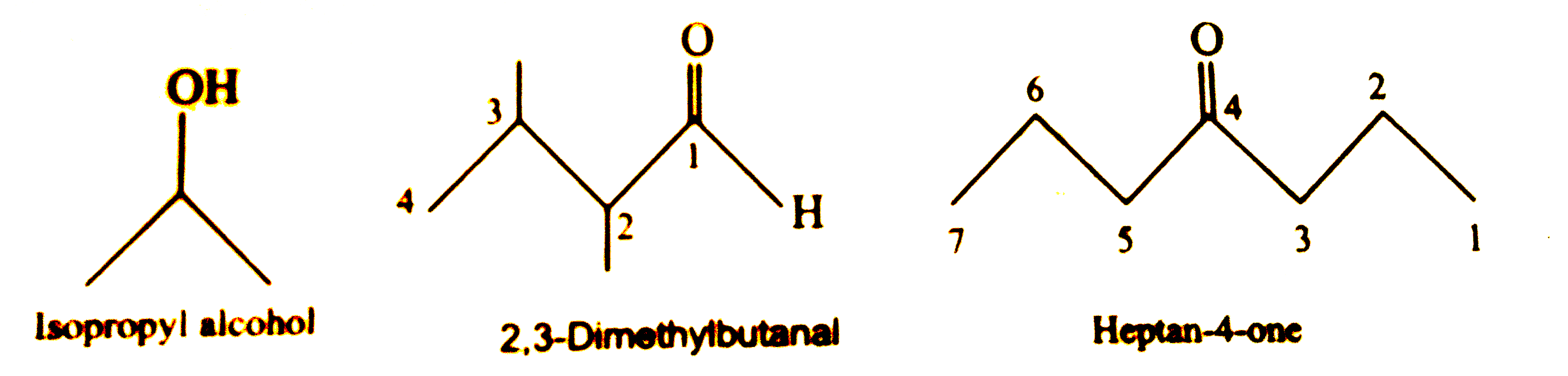
- Write bond line formulas for : (i) Isopropyl alcohol (ii) 2, 3-Dimet...
Text Solution
|
- Give the IUPAC name of the following compounds :
Text Solution
|
- Which of the following represents the corrcet IUPAC name for the compo...
Text Solution
|
- Draw the formulae for the first five numbers of each homologous series...
Text Solution
|
- Give condensed and bond line structural formulas and identify the func...
Text Solution
|
- Identify the functional groups in the following compouds :
Text Solution
|
- Which of the two: O(2)NCH(2)CH(2)O^(–) or CH(3)CH(2)O^(–) is expecte...
Text Solution
|
- Explain why alkyl groups act as electron donors when attacted to a pi-...
Text Solution
|
- Draw the resonance structures for the following compounds. Show the el...
Text Solution
|
- What are electrophiles and nucleophiles ? Explain with examples.
Text Solution
|
- Identify the reagents shown in bold in the following equations as nucl...
Text Solution
|
- Classify the following reactions in one of the reaction type studied i...
Text Solution
|
- What is the relationship between the members of following pairs of str...
Text Solution
|
- For the following bond cleavages, use curved-arrows to show the electr...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Give a brief description of the principles of the following techniques...
Text Solution
|
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|