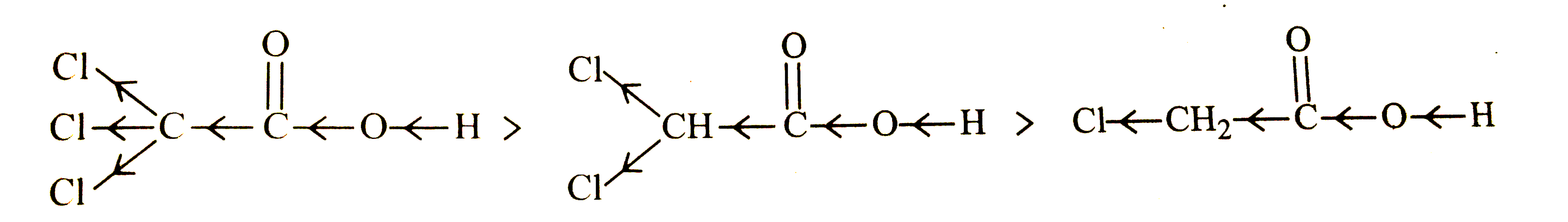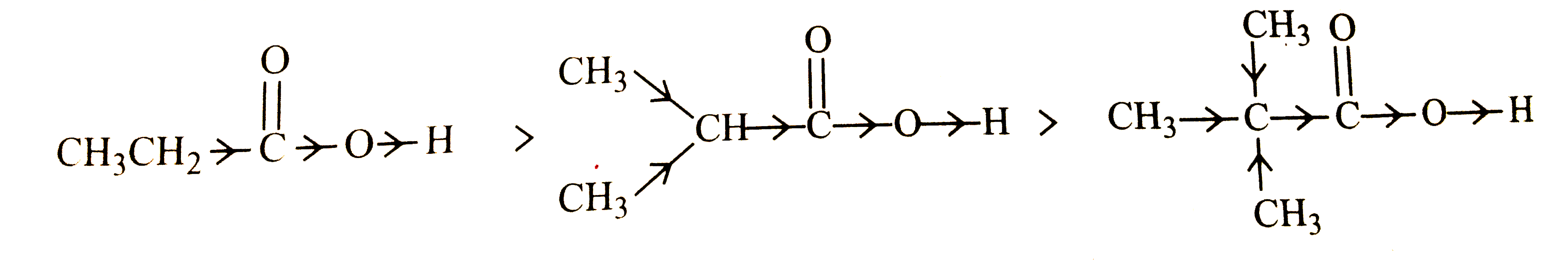Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - I)|15 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - II)|7 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Conceptual Questions (IV. Purification and Separation of Organic Compounds)|11 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-NCERT Questions and Exercises With Answers
- What is the relationship between the members of following pairs of str...
Text Solution
|
- For the following bond cleavages, use curved-arrows to show the electr...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Give a brief description of the principles of the following techniques...
Text Solution
|
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|
- Discuss the chemistry of Lassaigne’s test.
Text Solution
|
- Differentiate between the principle of estimation of nitrogen in an or...
Text Solution
|
- Discuss the principle of estimation of halogens, sulphur and phosphoru...
Text Solution
|
- Explain the principle of paper chromatography.
Text Solution
|
- Why is nitric acid added to sodium extract before adding silver nitrat...
Text Solution
|
- Explain the reason for the fusion of an organic compound with metallic...
Text Solution
|
- Name a suitable technique of the components from a mixture of calcium ...
Text Solution
|
- Explain why an organic liquid vaporises at a temperature below its boi...
Text Solution
|
- Will C Cl4 give white precipitate of AgCl on heating with nitrate? Giv...
Text Solution
|
- Why is solution of potassium hydroxide used to absorb carbon dioxide e...
Text Solution
|
- Acetic acid and not sulphuric acid is essential for acidification of s...
Text Solution
|
- An organic compound contains 69% carbon and 4.8% hydrogen, the remaind...
Text Solution
|
- A sample of 0.50 gm of an organic compound was treated according to Kj...
Text Solution
|
- 0.3780 g of an organic chloro compound gave 0.5740 g silver chloride i...
Text Solution
|