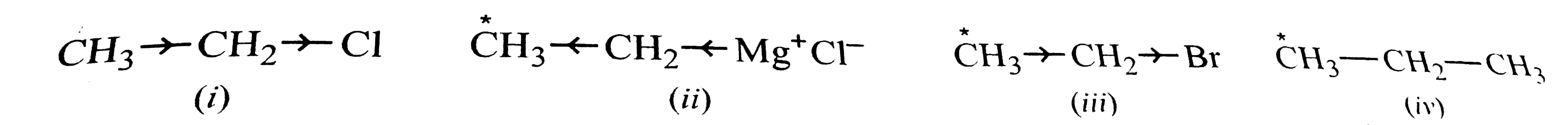A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - II)|7 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Short Answer Questions)|27 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise NCERT Questions and Exercises With Answers|40 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Multiple Choice Questions - I)
- Which of the following is the correct IUPAC name ?
Text Solution
|
- The IUPAC name for CH(3)-overset(O)overset(||)(C)-CH(2)-CH(2)-overs...
Text Solution
|
- The IUPAC name for
Text Solution
|
- Electronegativity of carbon atoms depends upon their state of hybridis...
Text Solution
|
- In which of the following, functional group isomerism is not possible ...
Text Solution
|
- The fragrnance of flower is due to the presence of some steam volatile...
Text Solution
|
- During hearing of a court case, the judge suspected that some changes ...
Text Solution
|
- The principle involved in paper chromatography is
Text Solution
|
- What si the correct order of decreasing stability of the following car...
Text Solution
|
- Correct IUPAC name for H(3)C - underset(C(2)H(5))underset(|)(CH) - un...
Text Solution
|
- In which of the following compounds the carbon marked with asterisk is...
Text Solution
|
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- Electrophilic additions reactions proceed in two steps. The first step...
Text Solution
|
- Covalent bond can undergo fission in two different ways. The correct r...
Text Solution
|
- The addition of HCl to an alkene proceeds in two steps. The first step...
Text Solution
|