Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Analytical Questions and Problems with Answers/Solutions (Problems)|4 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) I. Multiple choice Questions (With One Correct Answer)|135 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Additional Questions (Long Answer Type Questions)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-Analytical Questions and Problems with Answers/Solutions (Questions)
- Conversion of 1-bromobutane (I) to 2-bromobutane (II) is called isomer...
Text Solution
|
- The four hydrogens atoms of ethane lie in a plane. Do you think, four ...
Text Solution
|
- Explain why exists in keto form but exists in the enol form?
Text Solution
|
- Consider the following carbocations? Arrange them in decreasing o...
Text Solution
|
- With proper reasoning, arrange the following resonance structures in o...
Text Solution
|
- Iodide is a better nucleophile than bromide.Explain
Text Solution
|
- H(3)O^(+) or RN(4)^(+) neither acts as an electrophile nor as a nucleo...
Text Solution
|
- H(2)C = O or CH(3)CN acts as a nucleophile as well as an electrophile....
Text Solution
|
- Out of which will be eluted first in moderately polar solvent and wh...
Text Solution
|
- A compound which does not give a positive test in Lassaigne's test for...
Text Solution
|
- The 3D structure of a compound is given below : Write the 3D stru...
Text Solution
|
- A compound which does not give a positive test in Lassaigne's test for...
Text Solution
|
- Although C-D bond is stronger than C-H bond, yet (CH(3))(3)C^(+) (i) ...
Text Solution
|
- Although F has high -I effect, yet F(3)C^(+) is more stable . Explain ...
Text Solution
|
- Why less stable carbocations tend to rearrange to more stable carbocat...
Text Solution
|
- Although carbocations are always planar but carbanions and free radic...
Text Solution
|
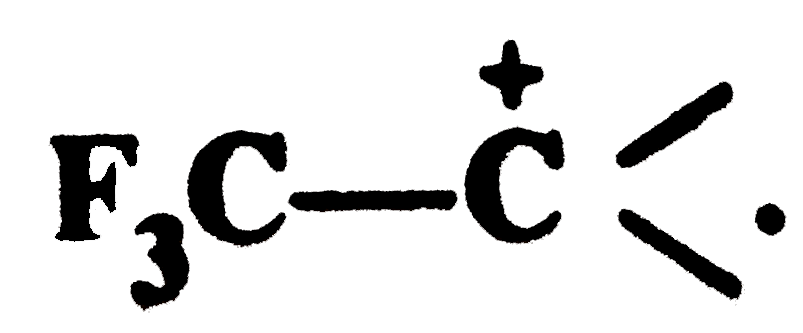 . Explain why is it so ?
. Explain why is it so ?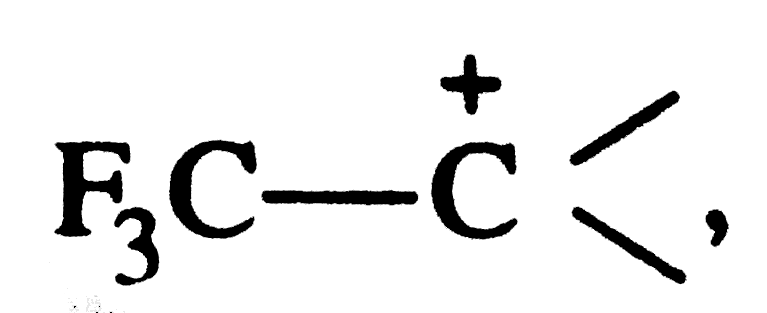 , the strongly electron withdrawing `Fe_(3)C-` group withdraws electrons of the C-C bond towards itself thereby intensifying the +ve charge and thus destabilising the carbocation.
, the strongly electron withdrawing `Fe_(3)C-` group withdraws electrons of the C-C bond towards itself thereby intensifying the +ve charge and thus destabilising the carbocation. 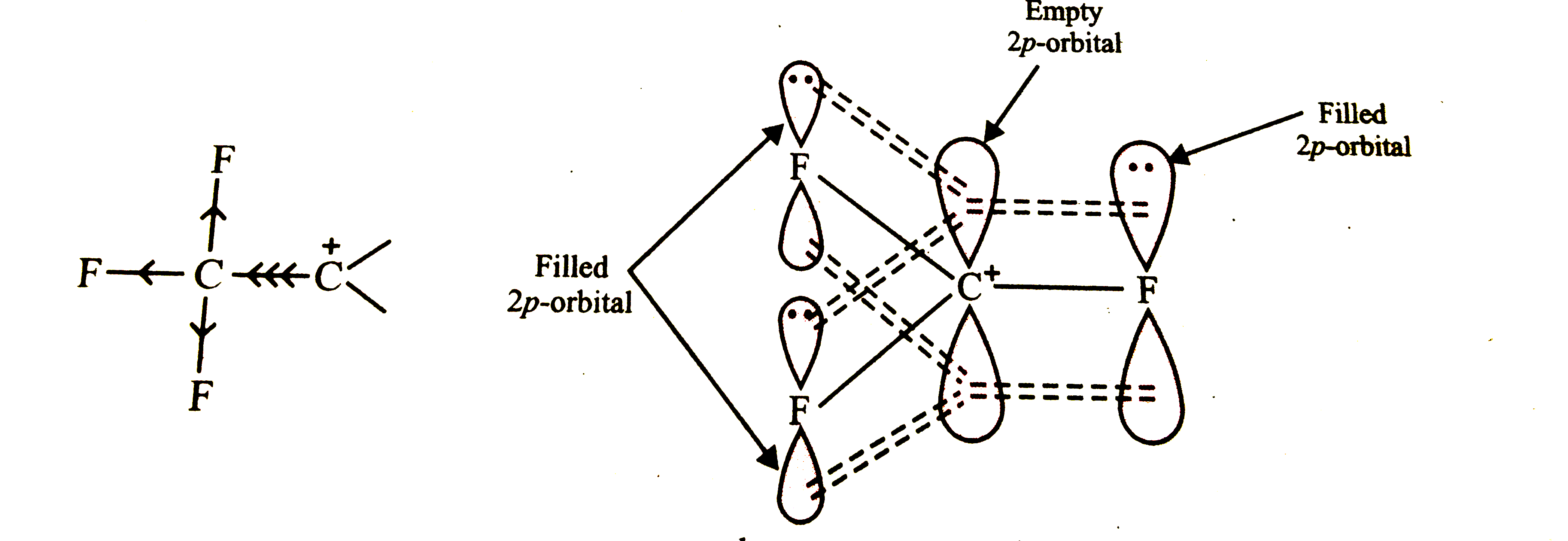
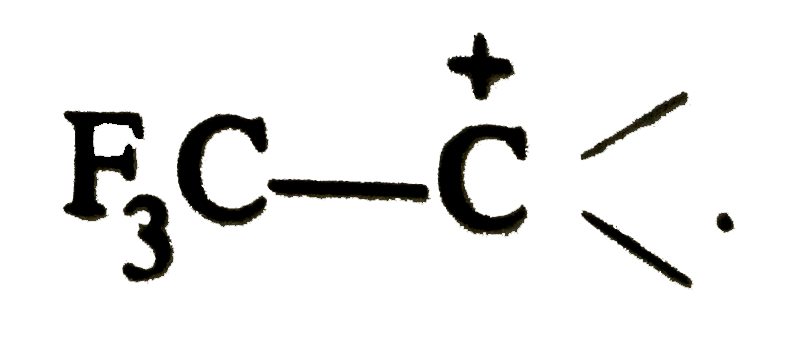 .
.