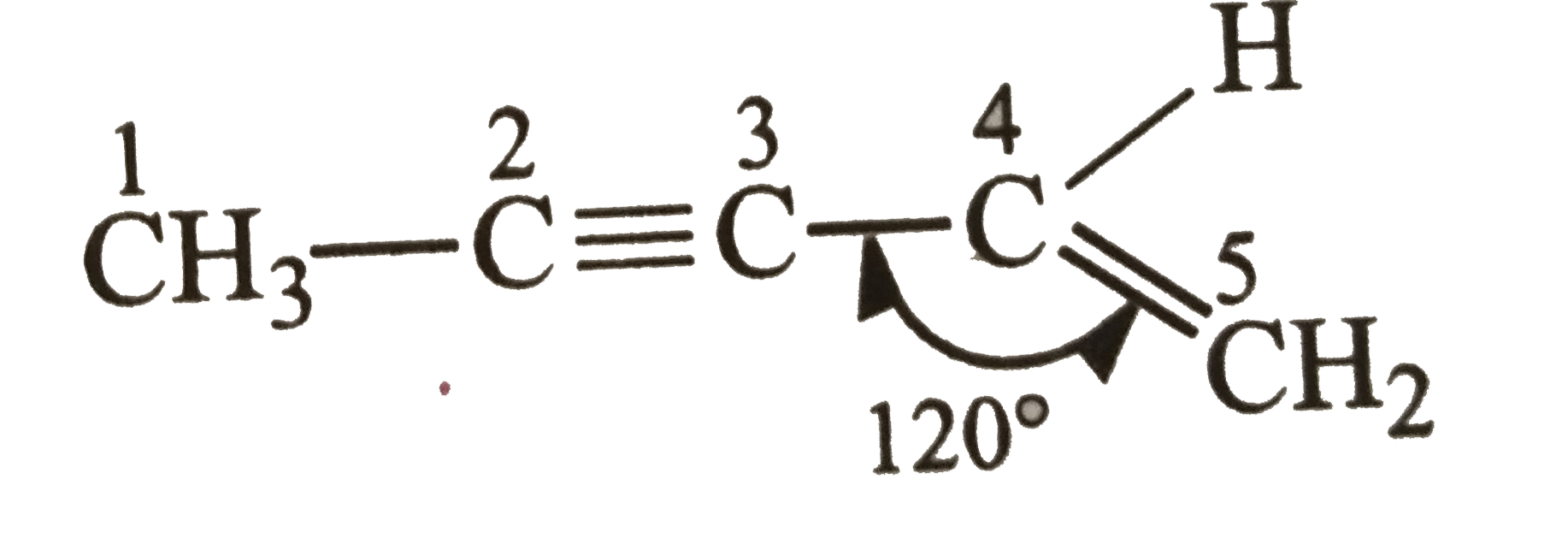A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) II. Multiple Choice Questions (with one or more than one correct answers)|8 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) III. Multiple Choice Questions (Comprehension)|12 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Analytical Questions and Problems with Answers/Solutions (Problems)|4 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-Competition Focus (Jee (Main and Advanced)/Medical Entrance) I. Multiple choice Questions (With One Correct Answer)
- In which of the following moleucles, all atoms are coplanar?
Text Solution
|
- Considering the state of hybridization of carbon atoms, find out the m...
Text Solution
|
- The maximum number of carbon atoms arranged linearly in the molecule, ...
Text Solution
|
- The number of sigma and pi-bonds present in 1, 3-butadiene are respect...
Text Solution
|
- In hexa-1,3-diene-5-yne, the number of C-C sigma, C-C pi and C-H sigma...
Text Solution
|
- The total number of pi-bond electrons in the following structure is
Text Solution
|
- The enolic form of ethyl acetoacetate as shown below has
Text Solution
|
- Which one of the following is a benzenoid aromatic compound ?
Text Solution
|
- Tropolone is an example of
Text Solution
|
- The structure of isobutyl group in an organic compound is :
Text Solution
|
- The IUPAC name for the hydrocarbons represented by the Swastik sign is
Text Solution
|
- Which isomer of hexane has only two different sets of structurally equ...
Text Solution
|
- A compound with molecular formula C(6)H(14) has two tertiary carbons. ...
Text Solution
|
- The IUPAC name of is
Text Solution
|
- The IUPAC name of the following molecule
Text Solution
|
- The correct IUPAC name for the compound is
Text Solution
|
- The IUPAC name of the following compound is
Text Solution
|
- What is the IUPAC nomenclature of the given compound ?
Text Solution
|
- Which one of the following is s-butylphenylvinyl methane ?
Text Solution
|
- Consider the following compound, IUPAC name of this compound is
Text Solution
|