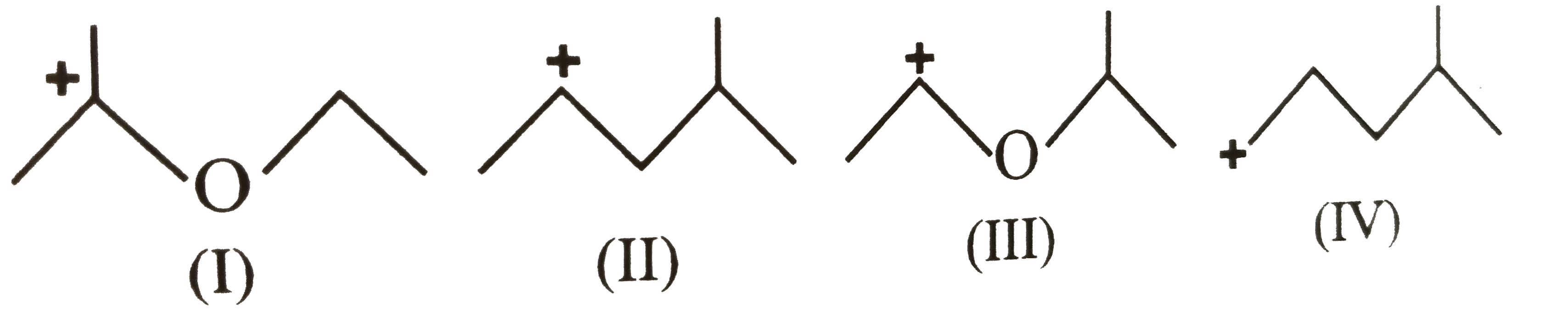A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) II. Multiple Choice Questions (with one or more than one correct answers)|8 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) III. Multiple Choice Questions (Comprehension)|12 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Analytical Questions and Problems with Answers/Solutions (Problems)|4 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-Competition Focus (Jee (Main and Advanced)/Medical Entrance) I. Multiple choice Questions (With One Correct Answer)
- Consider the following carbocation: (i) Cl(3)overset(+)C (ii) Cl(2...
Text Solution
|
- Which of the following cation would have greatest stability?
Text Solution
|
- The correct stability order for the following species is
Text Solution
|
- In which of the following compounds, the C-Cl bond ionisation shall gi...
Text Solution
|
- The decreasing order of stability of the carbonium ions C(6)H(5)CH(2...
Text Solution
|
- In the following carbocation, H//CH(3) that is most likely to migrate ...
Text Solution
|
- Consider the following carbocations I overset(+)underset((CH(3))(3)C...
Text Solution
|
- The order of stability of the following carbocations is underset(I)(...
Text Solution
|
- the ascending order of stability of the carbanion CH(3)(P),C(6)H(5)ove...
Text Solution
|
- Arrange the carbanions, (CH(3))(3)bar(C),bar(C)Cl(3),(CH(3))(2)bar(C)H...
Text Solution
|
- The stability of carbanions in the following. (i) RC -= C^(Θ) (ii)...
Text Solution
|
- The order of the base strength of the compounds (iii) NH(2)^(...
Text Solution
|
- Which of the following compounds prossesses the C-H bonds with the low...
Text Solution
|
- Most stable radical is
Text Solution
|
- The increasing order of stability of the following free radicals is :
Text Solution
|
- Arrange the following free radiacals in order of decreasing stability....
Text Solution
|
- The order of decreasing ease of abstraction of hydrogen atoms in the f...
Text Solution
|
- The reaction of methyl trichloroacetate (Cl(3)"CC"O(2)Me) with sodium ...
Text Solution
|
- For the following reactions: CH(3)CH(2)CH(2)Br + KOH rarr CH(3)CH=C...
Text Solution
|
- An alkyne combines with a conjugated diene to give an unconjugated cyc...
Text Solution
|