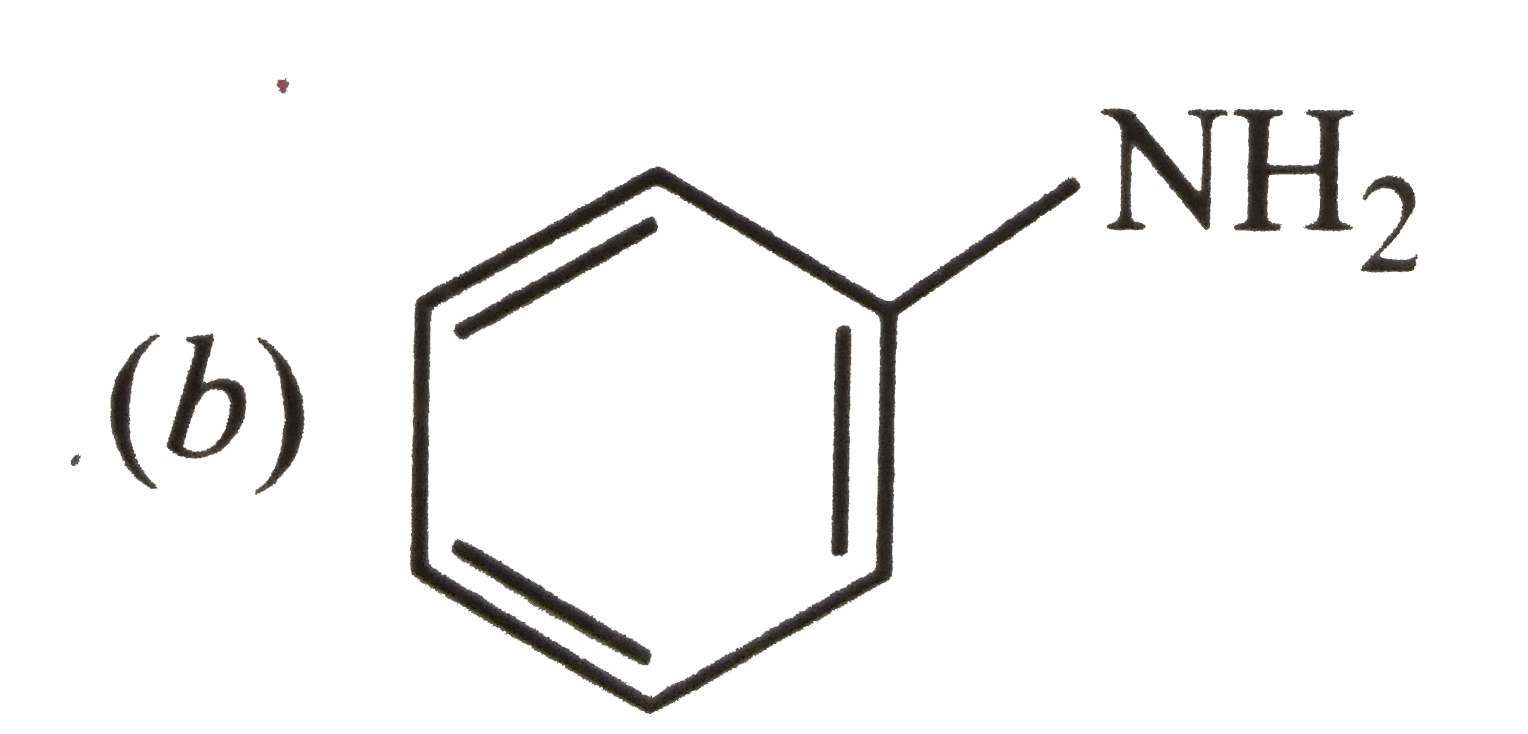
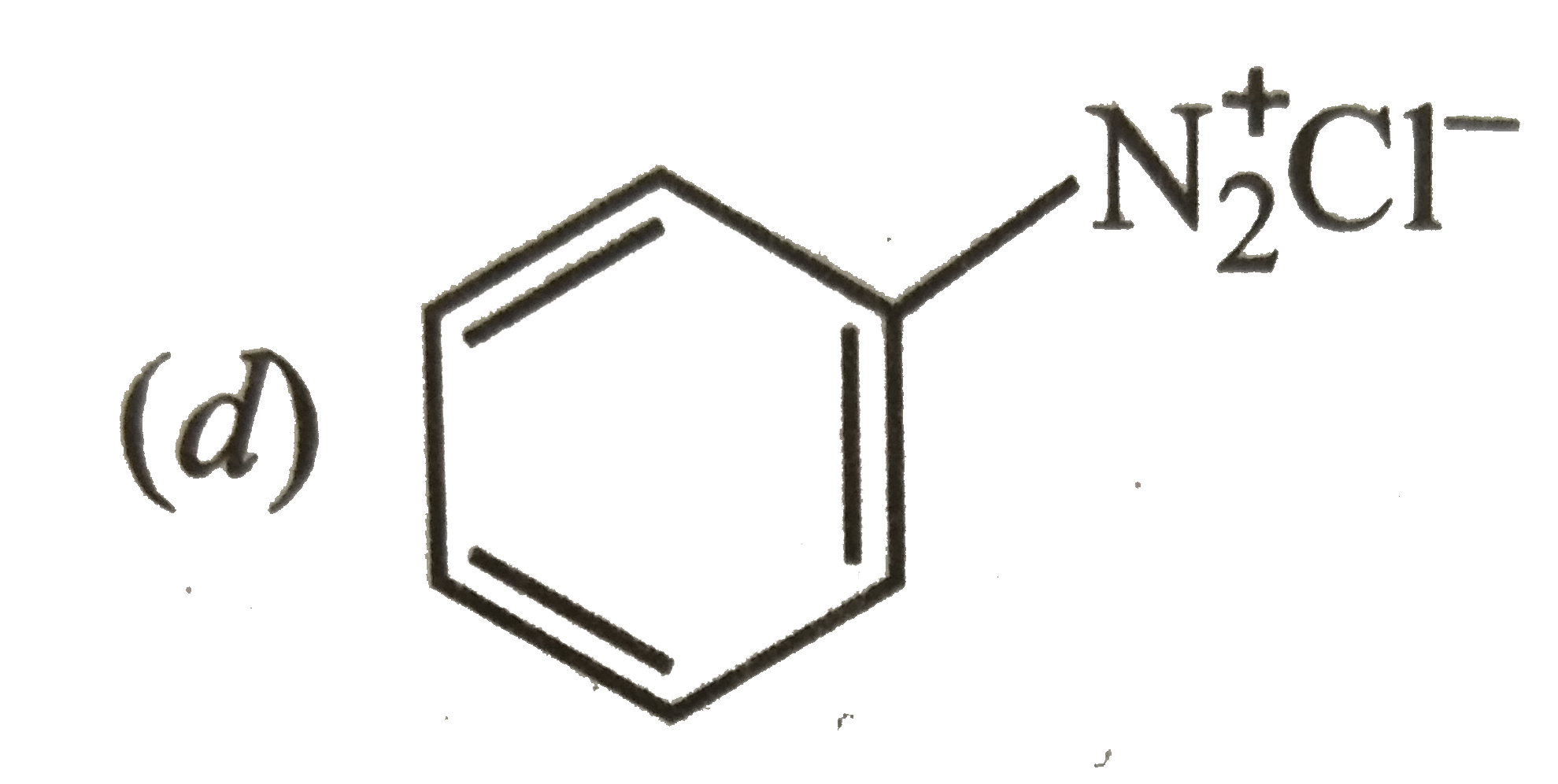
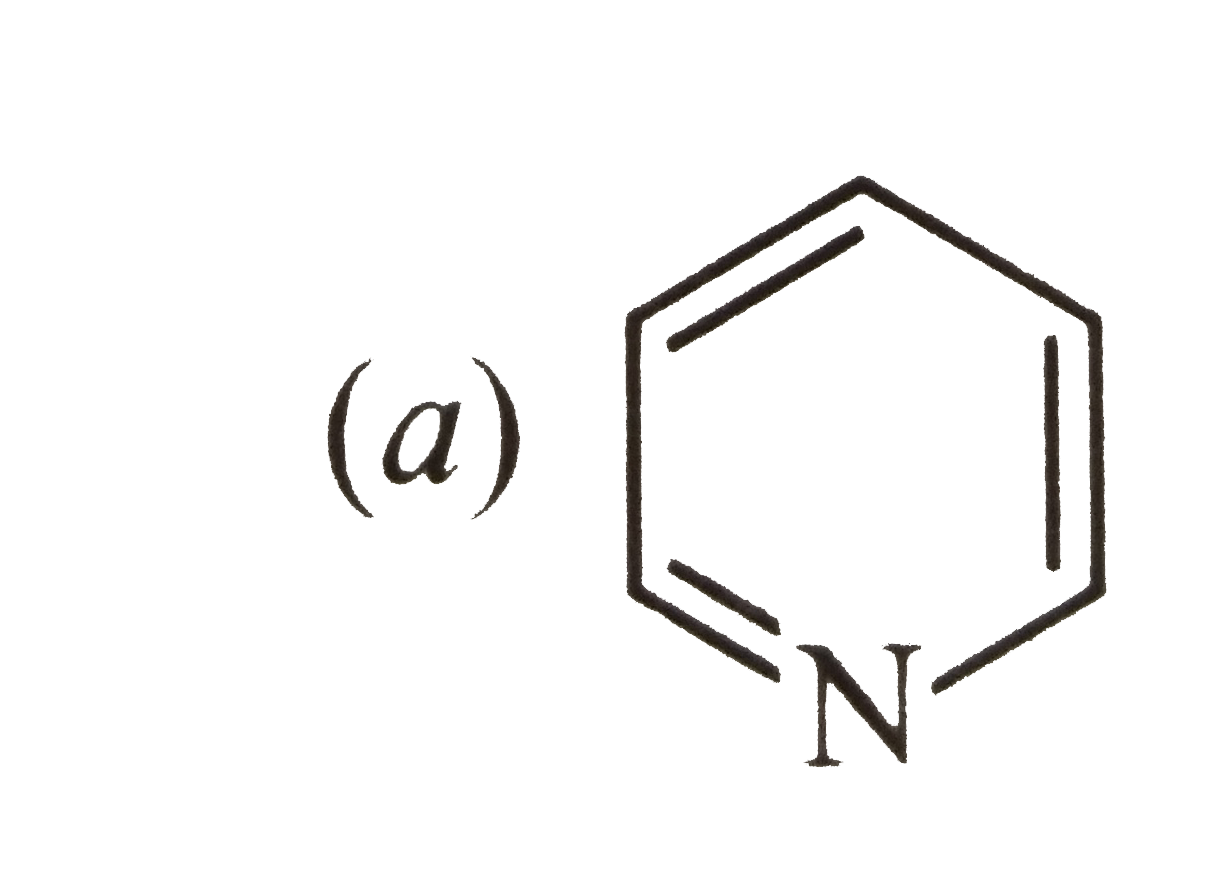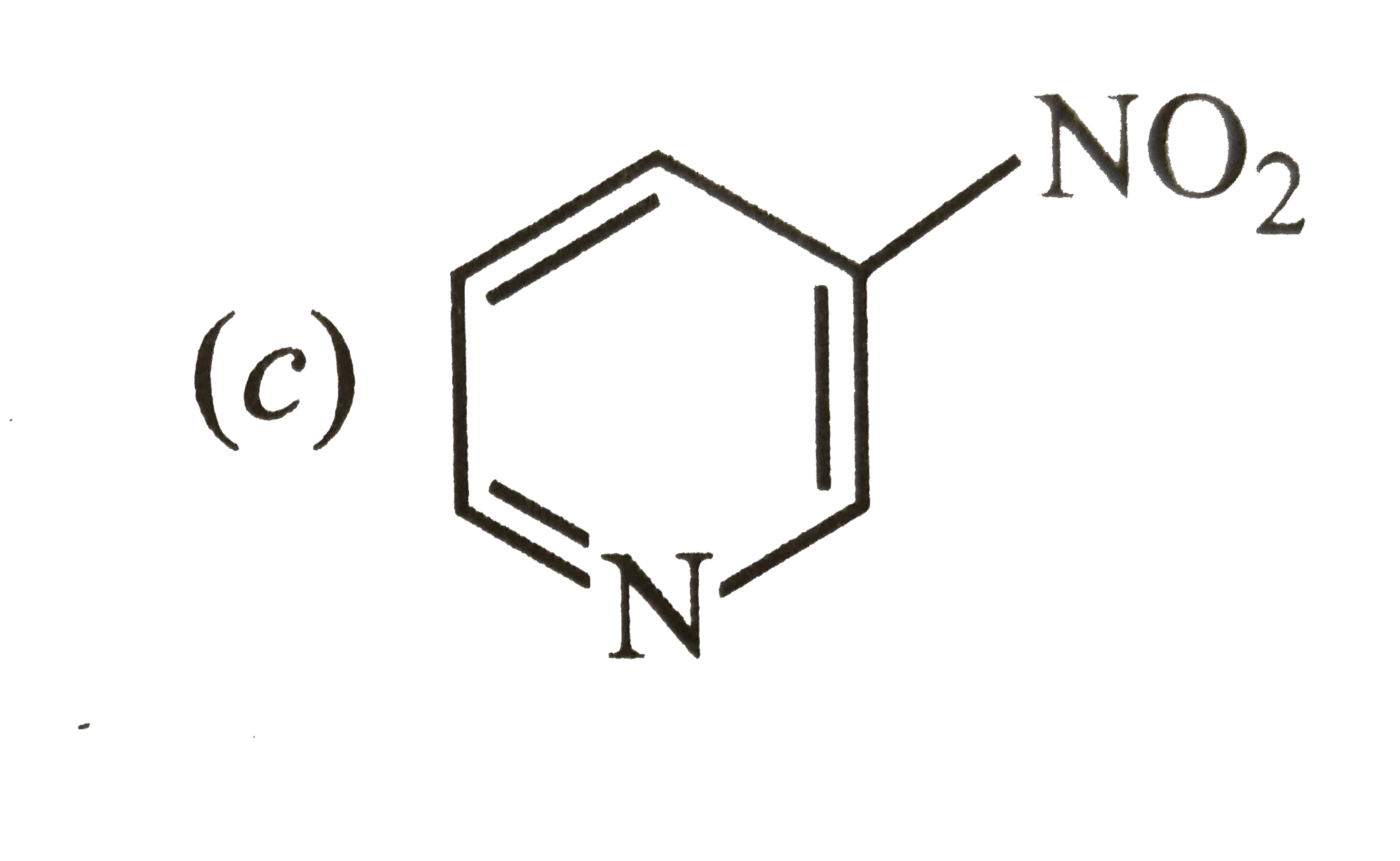A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) II. Multiple Choice Questions (with one or more than one correct answers)|8 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Competition Focus (Jee (Main and Advanced)/Medical Entrance) III. Multiple Choice Questions (Comprehension)|12 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES
PRADEEP|Exercise Analytical Questions and Problems with Answers/Solutions (Problems)|4 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 VideosP-BLOCK ELEMENTS (NITROGEN FAMILY)
PRADEEP|Exercise Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUES-Competition Focus (Jee (Main and Advanced)/Medical Entrance) I. Multiple choice Questions (With One Correct Answer)
- During the fusion of an organic compound with sodium metal, nitrogen o...
Text Solution
|
- In the Lassaigne's test for the detection of nitrogen in an organic co...
Text Solution
|
- The LaSSaigen's extract is boiled with conc. HNO(3) while testing for...
Text Solution
|
- Lassaigne's test (with silver nitrate) is commonly used to detect halo...
Text Solution
|
- Which of the following compounds will not give Lassaigne's test for ni...
Text Solution
|
- Which of the following compounds gives blood red colouration when its ...
Text Solution
|
- The colour of the solution/precipitate obtained in the elemental analy...
Text Solution
|
- Correct pair of compounds which gives blue colouration/precipitate and...
Text Solution
|
- 0.30g of an organic compound containing C, H, and O an combustion yiel...
Text Solution
|
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|
- In Duma's method for estimation of nitrogen. 0.25 g of an organic comp...
Text Solution
|
- Nitrogen can be estimated by Kjeldahl's method for which of the follow...
Text Solution
|
- In the Kjeldahl's method for estimation of nitrogen present in a soil ...
Text Solution
|
- The ammonia evolved from the treatment of 0.03 g of an organic compoun...
Text Solution
|
- 29.5 mg of an organic compound containing nitrogen was digested accord...
Text Solution
|
- A samlpe of 0.5 of an organic compound was analysed using Kjeldahl' me...
Text Solution
|
- For the estimation of nitrogen, 1.4 g of an organic compound was diges...
Text Solution
|
- In Carius method of estimation of halogens 250 mg of an organic compou...
Text Solution
|
- In the estimation of sulphur by Carius method, 0.480 f og an organic c...
Text Solution
|
- A gaseous hydrocarbon gives upon combustion, 0.72 g of water and 3.08 ...
Text Solution
|