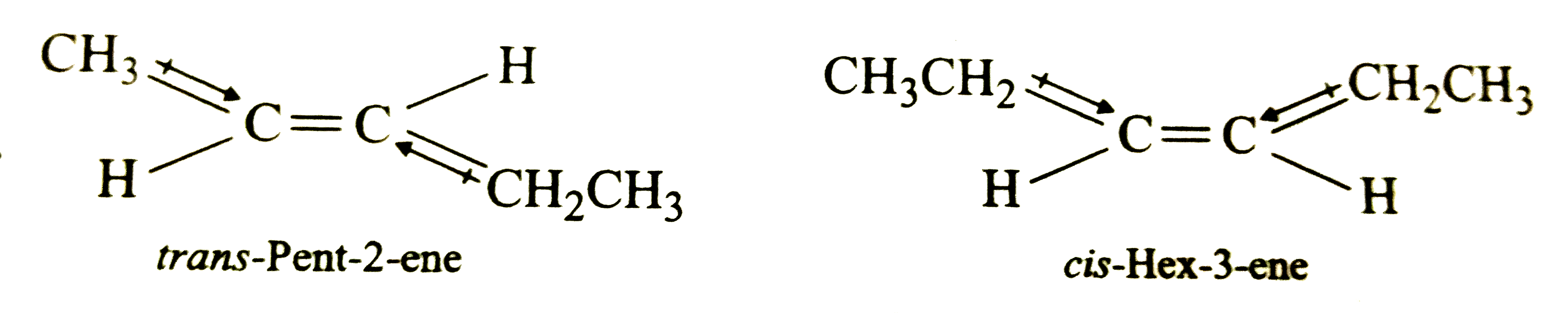A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (SHORT ANSWER QUESTIONS)|20 VideosHYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MATCHING TYPE QUESTIONS)|4 VideosHYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -I)|10 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -II)
- Some oxidation reactions of methane are given below. Which of them is/...
Text Solution
|
- Which of the following alkenes on ozonolysis give a mixture of ketones...
Text Solution
|
- Which are the correct IUPAC names of the following compound? H(3)C-C...
Text Solution
|
- Which are the correct IUPAC names of the following compounds ? ove...
Text Solution
|
- For an electrophilic substitution reaction , the presence of a halogen...
Text Solution
|
- In an electrophilic substitution reaction of nitrobenzene, the presenc...
Text Solution
|
- Which of the following are correct ?
Text Solution
|
- Four structures are given in options (a) to (d) . Examine them and sel...
Text Solution
|
- The molecules having dipole moment are :
Text Solution
|