Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise ADDITIONAL QUESTIONS (VERY SHORT ANSWER TYPE QUESTIONS )|30 VideosHYDROCARBONS
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|62 VideosHYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (ASSERTION AND REASON TYPE QUESTIONS)|4 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS )
- An alkyl halide C(5)H(11) (A) reacts with ethanolic KOH to give an alk...
Text Solution
|
- 448mL of a hydrocarbon (A) having C (87.80%), H (12.19%) weight 1.64g ...
Text Solution
|
- An unsaturated hydrocarbon 'A' adds two molecules of H(2) and on reduc...
Text Solution
|
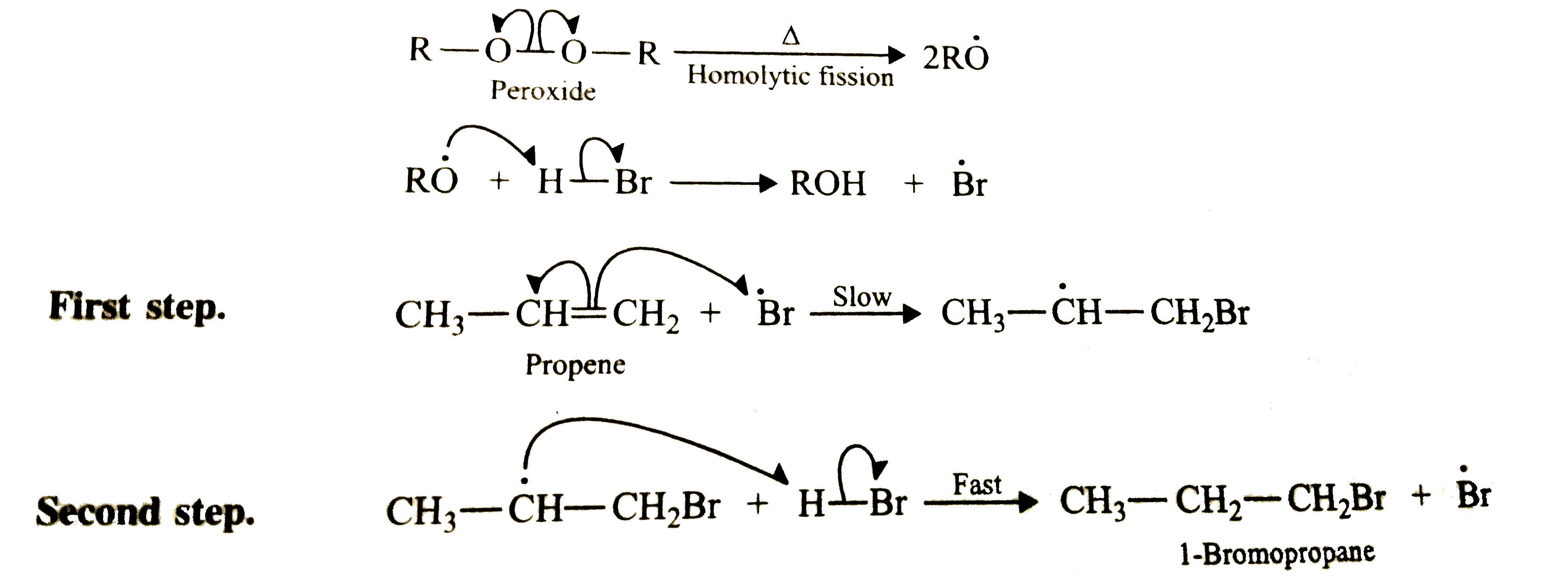
- In the presence of peroxide addition of HBr to propene takes place acc...
Text Solution
|